A recent survey on Author Perspectives on the Publishing System run by Editage Insights revealed that nearly half of all respondents had never heard of any of the industry-recognised Good Publication Practice standards.

A recent survey on Author Perspectives on the Publishing System run by Editage Insights revealed that nearly half of all respondents had never heard of any of the industry-recognised Good Publication Practice standards. Royal Society Publishing is committed to ensuring that all our papers are published responsibly and ethically and so we have created this handy little guide to the current Good Publication Practice Standards.
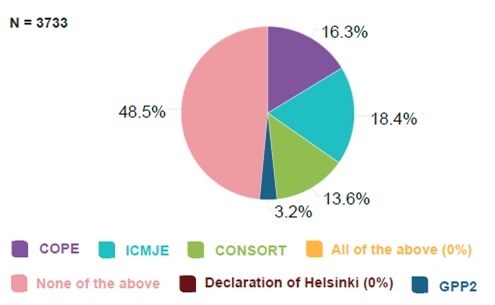
Percentage of Editage survey respondents who have heard of individual publication standards. Credit: Editage Insights
Why is it important?
Guidelines on good publication practice were developed to give editors and journals a framework to help them deal with the range of complex ethical issues that arise in scientific publishing. There are a number of organisations which have addressed a variety of issues to make sure authors can have faith in the peer review and editorial process, and readers can be assured of the quality of the science published in a journal. Below is a summary of the main organisations and their contributions to ensuring good publication practice.
COPE
Since its establishment in 1997 by three journal editors, the Committee on Publication Ethics (COPE) has gone from strength to strength and its membership has grown to over 12 000, including all journals published by the Royal Society. COPE has three main aims that they want to achieve:
- Provide practical resources to educate and support their members
- Provide leadership in thinking on publication ethics
- Offer a neutral, professional voice in current debates
In order to realise this, they provide a lot of support, primarily to journals and their editors to advise them on how to approach issues such as plagiarism, authorship disputes, fabrication of data and conflicts of interest. On their website they have a whole host of resources available to journal editors, publishers and authors, such as guidelines and flowcharts explaining the best practice for dealing with whatever issue you may have. There is also a forum where individual cases can be discussed openly to get specific advice on how to proceed. Perhaps one of the most helpful resources on the website is e-learning; this enables members to get essential training not readily accessible elsewhere for editors that are new to their role, so they can get educated on relevant ethical issues in publishing.
As part of this year’s Peer Review Week, COPE ran a webinar and also produced two new flowcharts to help peer reviewers in their work. They also contributed to LSE Impact blog.
ICMJE
The International Committee of Medical Journal Editors (ICMJE) recommendations were first published in 1978, mainly to address issues regarding differences in manuscript format and preparation across journals. However, in subsequent years the scope of the guidelines increased to include several issues in publishing that go beyond the original aims of the first publication. Unlike COPE, these guidelines are aimed primarily at authors looking to submit to ICMJE member journals, to help make the process as streamlined as possible and prevent unnecessary unsubmission due to formatting errors. It also sets out to clearly define the roles and responsibilities of the author and the journal, as well as providing guidelines regarding a variety of publishing and editorial issues including corrections, fees, misconduct and republication. These guidelines have been translated into nine languages.
CONSORT
The Consolidated Standards of Reporting Trials (CONSORT) is specifically aimed at helping researchers to report the findings of randomised controlled trials in a complete and transparent way by providing authors with a 25-item checklist and a flow diagram – both of which are available freely online. It was set up in 1993 by a group of 30 medical journal editors, clinical trialists, epidemiologists and methodologists, and merged with the Asilomar Working Group to publish the CONSORT Statement in 1996. It has since been revised twice, is endorsed by over 600 biomedical journals and significantly improves the peer review process by allowing reviewers to accurately judge the reliability and relevance of the findings being reported.
GPP3
The Good Publication Practice (GPP) guidelines were originally published in 2003 and have since been updated twice, resulting in the current GPP3 published in 2015. The GPP guidelines are focussed on ensuring responsible and ethical publication of clinical trials sponsored by pharmaceutical companies, with a renewed focus on transparency and high integrity. It is supported by the International Society for Medical Publication Professionals, which is an association of over 1500 members ranging from pharmaceutical companies, medical communication agencies and medical journal publishers and editors. It is committed to encouraging ethical and effective publication of medical research to inform treatment decisions. There are various resources available on the website to provide guidance on both general issues in publishing and also those more specific to the publication of clinical trial results.
Declaration of Helsinki
The Declaration of Helsinki was developed by the World Medical Association (WMA) in 1964 but has since been amended nine times, most recently in 2013. It provides guidelines on how to ethically conduct and publish medical research involving human subjects, human material and human data that may be identifiable. It is primarily aimed at physicians and aims to ensure that patient care is prioritised at all times when designing and undertaking a clinical trial. It covers topics ranging from risk, vulnerable groups and informed consent to scientific requirements, research ethics, and confidentiality.
Declaration of Taipei
The Declaration of Taipei was also developed by the WMA to address the relatively recent issue of health databases and biobanks. It was first adopted in 2002 and was revised in 2016. It provides ethical principles of the collection, storage and use of identifiable data and biological material after the care of the patient and is applied in conjunction with the Declaration of Helsinki. This is also aimed primarily at physicians and states all the rights of the individual involved.
There are a multitude of resources available to authors and publishers containing advice on how to navigate difficult ethical issues that may arise during publication. The commitment and work of these associations ensure that the publication process will continue to improve and become ever more streamlined and transparent. This can only aid the reproducibility of the data and contribute to the advancement of science through speedy and responsible dissemination of important results.
