Emerging technique provides further clues for nature’s champion of regeneration

What are the main points readers should take from the article?
CRG/SM: In our article, we implemented confocal Brillouin microscopy to study mechanical tissue properties in vivo in the axolotl. We focused on the skeletal tissue, particularly cartilage, in the forearm and digits.
Particular points we hope readers take from our work are:
First, tissue mapping with Brillouin microscopy reveals a distinct mechanical phenotype when applied to axolotl skeletal digits, in which a collagen rich extracellular matrix (ECM) contrasts cell-containing domains. Second, tissue mechanical properties are dynamically regulated and provide additional information complementing prevailing, biological and biochemical assays. In particular, we demonstrated how the mechanical properties of cartilage change during development and regeneration. Our analysis of morphological features suggested an advanced stage in the regenerative process. The mechanical information obtained by Brillouin microscopy, however, revealed that the recovery of mechanical tissue properties was delayed in comparison to the recovery of morphological features. This suggests that the overall repair process involves also a mechanical regeneration, and that mechanical tissue properties do not merely mirror the presence of certain morphological features. We believe our work underscores the need to further explore mechanical aspects accompanying repair processes. Brillouin microscopy will play an important role in further increasing our current knowledge in this field. We also hope this Methods and Techniques article will provide inspiration for other studies aiming to complement biochemical analyses with mechanical measurements.
Why were axolotl chosen as the model?
CRG/SM: Axolotls are a wonderful model for studying regeneration in vertebrates. They regenerate various body parts, such as the limb, tail and gills. Technological advances that emerged during the last decades (e.g. transgenesis, RNA-seq) have allowed us to study the biochemical mechanisms underlying the regenerative processes in the axolotl in great detail. This information can be used to complement mechanical investigations, perhaps even shed light on what determines mechanical features in a distinct process and type of tissue. Additionally, axolotls are naturally transparent during juvenile stages, which permits in vivo imaging of the regenerative process. All these features, technologies, and the research of many scientists have elevated the axolotl to a key model system for regeneration.
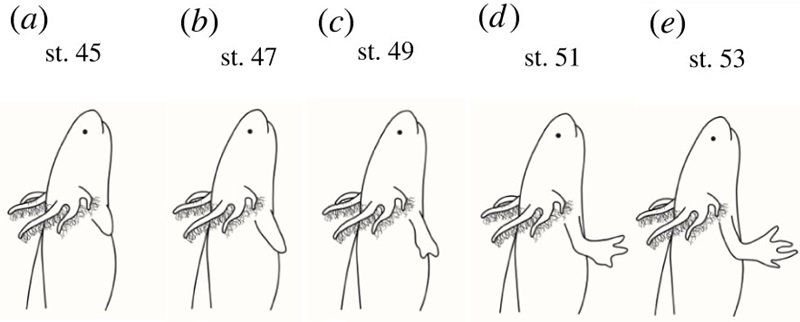
Extracted from Figure 3 - schematic representation of limb development stages DOI: 10.1098/rsob.220078
What is special about the technique used?
SM: Brillouin microscopy offers a non-invasive, contact- and label-free investigation of mechanical cell and tissue properties. Due to these features, it is particularly suited to quantify mechanical material properties under physiological conditions, i.e. inside living specimens. Most other techniques that can be used to quantify mechanical properties of tissues either rely on physical contact between a load-applying probe or lack sub-cellular resolution. In those cases, we would have to isolate the tissue of interest from its natural environment, or we would not get a similarly resolved spatial information. Most importantly, Brillouin microscopy enables repeated measurements within the same specimen to capture potentially dynamic mechanical processes. Thereby, it supports an experimental strategy that aims to maximize the information obtained per animal and facilitates a drastic reduction of the number of experimental animals.
What was most surprising finding in the study?
CRG/SM: We were definitively surprised by the high contrast of the Brillouin frequency shift between the collagen-rich ECM and the engulfed cells, and, of course, that the regeneration of the mechanical tissue properties appeared to be delayed with respect to the presence and recovery of morphological features. To us, it appears as if the mechanical tissue properties go through a maturation process that gradually approaches the original mechanical phenotype only after tissue structures are re-instated. Overall, we were really excited about the added value that Brillouin microscopy brings to axolotl regeneration. We believe our work will be useful to other researchers thinking about how tissue and cell mechanics are influencing the process of limb regeneration not only in salamanders.
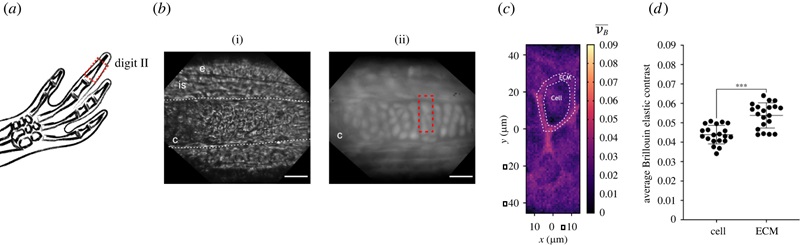
Confocal Brillouin microscopy maps cartilage architecture, identifying differences between chondrocytes and ECM (Figure 1, DOI: 10.1098/rsob.220078)
What's next for you and your research?
TSG: After seeing the findings of this publication, we are excited to continue exploring how individual cells interpret the mechanical signals, and how this is translated into the collective response. We know that the microenvironment where a cell resides is important for the type of response this will elicit. Is a cell in a constrained environment responding to a molecular signal in the same way a cell in a looser environment would? We know little about how repair and regeneration differ in different locations of the body, and we are starting to explore just that. It is exciting to think that with this technique, we can study intact cells in a multi-tissue structure like the limb, through time and preserving their physical contacts.
Do you have an exciting new discovery that you would like to publish in Open Biology? Find out more about our author benefits and submission process.
Image credits: Top image, Leucistic Axolotl, Wikimedia Commons, CC-BY 3.0; Figures, Tatiana Sandoval-Guzmán (10.1098/rsob.220078).



