Links to external sources may no longer work as intended. The content may not represent the latest thinking in this area or the Society’s current position on the topic.
Cells: from Robert Hooke to Cell Therapy – a 350 year journey
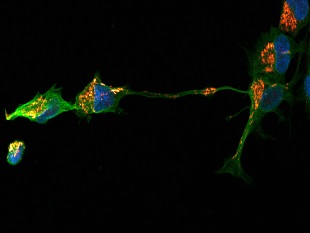
Scientific discussion meeting organised by Professor Sir Ian Wilmut FRS, Professor Johan Hyllner and Professor Chris Mason
Event details
The importance of cellular structure was first recognised by the British scientist Robert Hooke and described in Micrographia (Royal Society, September 1665). In this meeting, world-leading researchers will describe the way in which new approaches to cell therapy are being provided by our progressively greater understanding of the biology of cells, in particular different populations of stem cells.
The meeting programme can be downloaded here.
A theme issue of Philosophical Transactions B has been published in association with this meeting.
Attending this event
This event is intended for researchers in relevant fields and is free to attend. There are a limited number of places and registration is essential. An optional lunch is offered and should be booked during registration (all major credit cards accepted).
Enquiries: Contact the events team
Organisers
Schedule
Chair

Professor Roger Pedersen, University of Cambridge, UK

Professor Roger Pedersen, University of Cambridge, UK
| 09:05 - 09:35 |
Robert Hooke, microscopy, and the cell in early science
In 1665 Robert Hooke published Micrographia, the world's first fully-illustrated book of microscopy, and revealed to his readers a completely new world. Along with images of insect, mineral and plant specimens he included a section of cork, describing the tiny box-like structures he observed as 'cells'. Although he did not understand their biological function fully, he attributed to them some of the properties of cork such as buoyancy. In Micrographia, Hooke had two goals: to present his observations, and to persuade his readers that scientific research had the potential to improve people’s lives. Hooke made a point of linking his observations with his readers’ experiences of everyday life – for example, including an enormous image of a louse clinging to a human hair, rather than simply showing the insect alone on the page. By investigating the microscopic world, Hooke hoped to understand the mechanisms and structures of nature and perhaps make use of them in his own instruments and inventions. This talk will show that Hooke’s microscopic research underpinned his theories of the natural world, including his conviction that fossils were the remains of once-living plants or animals, a theory that would only be widely accepted over a century after Hooke’s death. 
Dr Felicity Henderson, University of Exeter, UK

Dr Felicity Henderson, University of Exeter, UKDr Felicity Henderson is a Lecturer in Archives and Material Culture at the University of Exeter. She is co-investigator on the AHRC-funded project 'Making Visible: the Visual and Graphic Practices of the Early Royal Society' (2015-2019). Her current research focusses on the social and intellectual practices that underpinned the activities of the early Royal Society Fellows, and she is editing a new edition of the Diary of Robert Hooke for publication with Oxford University Press. She has previously published on topics including early-modern linguistics and science, scientific translation, and seventeenth-century university satire. |
|---|---|
| 09:50 - 10:20 |
Evolution of Normal and Neoplastic Tissue Stem Cells
The transition from single cell species to multicellular organisms led to organ and tissue development, which has resulted in the emergence of specialized cells that self-renew as well as differentiate, called stem cells. Following embryonic development, most tissues and organs are continuously regenerated from tissue/organ specific stem cells. In most tissues only the primitive stem cells self-renew. Within a tissue, stem cells may be diverse, and subject to stem cell competition for their niche. Both germline stem cell and somatic stem cell competition in species as diverse as colonial protochordates and humans have been documented. Stem cell isolation and transplantation is the basis for regenerative medicine. Self-renewal is dangerous, and therefore strictly regulated. Poorly regulated self-renewal can lead to the genesis of cancer stem cells, the only self-renewing cells in the tumor. Dr Weissman and colleagues have followed the progression from hematopoietic stem cells (HSCs) to myelogenous leukemias. It was found that the preleukemic clones progress at the stage of HSCs, from which emerge “leukemia” stem cell at an oligolineage downstream stage that has evaded programmed cell death [PCD] and programmed cell removal [PrCR], that self-renews. In early chronic myeloid leukemia, bcr-abl+ HSC clones outcompete normal HSCs. The transition from the chronic phase to myeloid blast crisis results in the leukemia stem cells appearing in the granulocyte-macrophage progenitor (GMP) stage. While there are many ways to defeat PCD and senescence, there appears to be one dominant method to avoid PrCR—the expression of cell surface “don’t eat me” CD47, the ligand for macrophage SIRPα. All cancers tested express CD47 to overcome expression of “eat me” signals such as calreticulin. Antibodies that block the CD47–SIRPα interaction enable phagocytosis and killing of the tumor cells in vitro and in vivo. All tested human cancers are susceptible to phagocytosis in the presence of anti-CD47 blocking antibodies, which are in clinical trials. 
Professor Irving Weissman, Stanford University, USA

Professor Irving Weissman, Stanford University, USAIrving L Weissman, MD, is the Director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine and Director of the Stanford Ludwig Center for Cancer Stem Cell Research. Professor Weissman co-founded, was a Director, and chaired the Scientific Advisory Board at SyStemix (1988-1996), StemCells Inc. (1996-present), and Cellerant (2001-2009). His research encompasses the biology and evolution of stem cells and progenitor cells, mainly blood-forming and brain-forming. He is also engaged in isolating and characterizing the rare cancer and leukemia stem cells as the only dangerous cells in these malignancies, especially with human cancers. Finally, he has a long-term research interest in the phylogeny and developmental biology of the cells that make up the blood-forming and immune systems. His laboratory was first to identify and isolate the blood-forming stem cell from mice, and has purified each progenitor in the stages of development between the stem cells and mature progeny (granulocytes, macrophages, etc). |
| 11:05 - 11:35 |
Neural stem cells: their role in brain development, maintenance and potential nervous system therapies
The central nervous system (CNS) is the most complex of tissues, with hundreds of types of neurons and glia patterned into unique regions, connected through intricate, complex circuits. Developmental studies have shown that the initial plate of neuroepithelial cells is regionally patterned by morphogenic signals. This areal identity is then interpreted by neural stem cells and more restricted precursor cells, collectively called neural progenitor cells (NPCs), to produce the diverse cells of each CNS region. Progeny arise from NPCs on a defined schedule, and migrate to their final position. The birthdate of each cell type is precise for a given species, but varies widely, e.g. from mice to men. Studies of these developmental events have revealed numerous classes of NPCs. Some are present just through the developmental period, others are retained in active stem cell zones throughout life. Notably, in the hippocampal dentate gyrus, stem cells continually make new neurons that contribute to memory formation. Adult progenitors capable of producing oligodendrocytes and astrocytes are also present widely in the CNS. Neurodegenerative diseases typically attack specific CNS populations, for example, the hippocampal stem cell system in Alzheimer’s Disease, and midbrain dopaminergic neurons in Parkinson’s disease. Traumatic damage can cause loss of numerous cell types. Human NPCs are being harnessed to replace the lost cells and are already in clinical trials. In addition, the fact that progenitor cells are present in the adult CNS offers the exciting opportunity for stimulating endogenous reparative processes, reawakening the inner salamander. 
Dr Sally Temple, Neural Steme Cell Institute, USA

Dr Sally Temple, Neural Steme Cell Institute, USASally Temple, PhD, is the Co-Founder and Scientific Director of the Neural Stem Cell Institute located in Rensselaer, NY, USA. Dr Temple’s group is focused on studies of neural stem cells, and using this knowledge to develop therapies for central nervous system disorders. Dr Temple trained at Cambridge University and University College London with Dr Martin Raff FRS. In 1989, Dr Temple discovered that the embryonic mammalian brain contained a rare, multipotent stem cell that could be grown in tissue culture, producing both neurons and glia. Since then, her group has continued to make pioneering contributions to the field of neural stem cell research, identifying cell-intrinsic and extracellular niche factors that participate in their self-renewal and differentiation into diverse cell types. In recognition of her work, Dr Temple has received the Royal Society Stothert Research Fellowship, the Javits NIH merit award, the MacArthur award and the Ellison investigator award. Dr Temple is currently the president-elect of the International Society for Stem Cell Research. |
| 11:50 - 12:20 |
Somatic cell nuclear transfer: origins, the present position and future opportunities
Nuclear transfer involves the transfer of the nucleus from a donor cell into an egg from which the chromosomes have been removed was considered first as a means of assessing changes during development in the ability of the nucleus to control development. In 1996 a nucleus from an adult cell controlled development to term of Dolly the sheep. She was the first adult clone derived by transfer of a nucleus from an adult cell. The new procedure has been used to target precise genetic changes into livestock; however, the greatest inheritance of the Dolly experiment was to make biologists think differently. If unknown factors in the recipient egg could “reprogramme” the nucleus to a stage very early in development then there must be other ways of making similar changes. Within 10 years two laboratories showed that the introduction of selected proteins changed some of the cells to stem cells equivalent to those derived from an embryo. This ability is providing revolutionary new opportunities in research and cell therapy. 
Professor Sir Ian Wilmut FRS, MRC Centre for Regenerative Medicine, UK

Professor Sir Ian Wilmut FRS, MRC Centre for Regenerative Medicine, UKIan Wilmut is the Director of the MRC Centre for Regenerative Medicine at the University of Edinburgh. The Mission of the Centre is to develop new treatments for human disease through innovative research with stem cells. The new Centre covers the full spectrum of research - from basic mechanisms of stem cell regulation, via rigorous translational studies, to clinical trials with stem cells and their derivatives. Purpose designed facilities that will be completed in spring 2011 are being built alongside the new Royal Infirmary of Edinburgh. The research of Ian’s own group is directed toward understanding the mechanisms that bring about reprogramming of nuclei and with exploiting new opportunities for reprogramming cells to study degenerative diseases, such as motor neuron disease. |
Chair

Professor Fiona Watt FMedSci FRS, King's College London, UK

Professor Fiona Watt FMedSci FRS, King's College London, UK
Fiona Watt obtained her first degree from Cambridge University and her DPhil, in cell biology, from the University of Oxford. She was a postdoc at MIT, where she first began studying differentiation and tissue organisation in mammalian epidermis. She established her first research group at the Kennedy Institute for Rheumatology and then spent 20 years at the CRUK London Research Institute (now part of the Francis Crick Institute). She helped to establish the CRUK Cambridge Research Institute and the Wellcome Trust Centre for Stem Cell Research and in 2012 she moved to King's College London to found the Centre for Stem Cells and Regenerative Medicine. Fiona Watt is a Fellow of the Royal Society, a Fellow of the Academy of Medical Sciences and an Honorary Foreign Member of the American Academy of Arts and Sciences. Her awards include the American Society for Cell Biology (ASCB) Women in Cell Biology Senior Award, the Hunterian Society Medal and the FEBS/EMBO Women in Science Award. In 2016 she was awarded Doctor Honoris Causa of the Universidad Autonoma de Madrid.
| 13:35 - 14:05 |
Present and future challenges of pluripotent stem cells
The old is going destiny for us. However mature differentiated cells making up our body can be rejuvenated to embryo-like fate called pluripotency which is an ability to differentiate into all cell types by enforced expression of defined transcription factors. The discovery of this induced pluripotent stem cell (iPSC) technology has opened up unprecedented opportunities in regenerative medicine, disease modeling and drug discovery. In this talk, Dr Takahashi will introduce the process leading to iPSC technology, applications and future perspectives of human iPSCs. And, also show that how iPSC technology has evolved along the way. 
Dr Kazutoshi Takahashi, Gladstone Institute, USA

Dr Kazutoshi Takahashi, Gladstone Institute, USADr. Kazutoshi Takahashi is a principal investigator of Center for iPS cell research and application at Kyoto University, Japan, and a visiting scientist of Gladstone Institute at University of California San Francisco. He received his PhD from the Nara Institute of Science and Technology, Japan in 2005 under the supervision of Professor Shinya Yamanaka. Then he discovered that the set of four transcription factors can induce cell fate conversion of somatic cells to pluripotent state. Resulting induced Pluripotent Stem cells (iPSCs) have opened new avenues for regenerative medicine, disease modeling and drug discovery. He received domestic and international prizes such as ISSCR McEwen Centre Award for Innovation and NYSCF Robertson Stem Cell Prize. |
|---|---|
| 14:20 - 14:50 |
Direct reprogramming towards the neural lineage
Cellular differentiation and lineage commitment are considered robust and irreversible processes during development. Challenging this view, we found that expression of only three neural lineage-specific transcription factors Ascl1, Myt1l, and Brn2 could directly convert mouse fibroblasts into functional in vitro. These induced neuronal (iN) cells expressed multiple neuron-specific proteins, generated action potentials, and formed functional synapses. Thus, iN cells are bona fide functional neurons. Addition of a fourth transcription factor Neurod1 also enabled reprogramming of human fibroblasts. Completely unexpectedly, the iN cell reprogramming process is substantially more efficient and faster than reprogramming to pluripotent stem cells. By investigating the mechanism of action of the transcription factors involved, we found a molecular explanation for this discrepancy. We found that Ascl1, one of the key driving iN cell transcription factors, is not only a pioneering factor in the sense that it can bind nucleosomal DNA in the fibroblast chromatin context, but we observed it bound to its physiological targets two days after infection in mouse fibroblasts as defined as targets in neural stem and progenitor cells. The three main iPS cell reprogramming factors Oct4, Sox2, and Klf4, on the other hand, have been shown to bind nucleosomal DNA but mostly bind ectopic genomic sites that are typically not bound by them in ES cells. Thus, the specific properties of the transcription factors used for reprogramming and their interaction with the chromatin appear to determine the reprogramming dynamics and efficiency between reprogramming systems. We also found that expression of neuronal lineage determining factors in human ES and iPS cells yields functionally mature neurons of unprecedentedly speed. These “ES-iN cells” are in quality almost equivalent to a primary neuronal culture and we have shown to be useful to study synaptic phenotypes of disease-associated mutations. 
Dr Marius Wernig, Institute for Stem Cell Biology and Regenerative Medicine, Stanford University, USA

Dr Marius Wernig, Institute for Stem Cell Biology and Regenerative Medicine, Stanford University, USAMarius Wernig was born in Innsbruck, Austria. Early on he was exposed to local and contemporary music, which inspired him to not only actively perform musical instruments but also compose. He studied with the contemporary composers David Graham and Stefan Hakenberg. Some of his work was performed by prestigious venues such as the Cologne Opera House or the Bonn Beethoven Orchestra. Despite these early successes, he then decided to study medicine at the University of Vienna. After completion of the basic medical sciences he continued the clinical studies at the Technical University of Munich, Germany. There he joined the laboratory of Rudi Balling to perform his Ph.D. thesis on the effects of valproate on skeletal development and the mechanism of its teratogenicity. After graduation, he began a residency programme in neuropathology at the University of Bonn, Germany, under the supervision of Otmar D. Wiestler. At that institute, he joined the laboratory of Oliver Brüstle working on neural differentiation of embryonic stem cells. For his postdoctoral training, Marius Wernig worked in the laboratory of Rudolf Jaenisch at the Whitehead Institute, MIT, where he spearheaded one of the few research groups worldwide to successfully create pluripotent stem cells from mouse skin cells. He was recently recruited to the faculty of the Institute for Stem Cell Biology and Regenerative Medicine and the Department of Pathology at Stanford University. His laboratory at Stanford is working on the epigenetic regulation of stem and somatic cells. For instance, his laboratory recently isolated defined factors that induce the direct conversion of skin fibroblasts into functional neurons. |
| 15:30 - 16:00 |
Naive pluirpotency

Professor Austin Smith, FRS, FRSE, Wellcome Trust – Medical Research Council, Cambridge Stem Cell Institute, University of Cambridge

Professor Austin Smith, FRS, FRSE, Wellcome Trust – Medical Research Council, Cambridge Stem Cell Institute, University of CambridgeProfessor Smith obtained his Ph.D. from the University of Edinburgh in 1986. Following postdoctoral research at the University of Oxford, he joined the Institute for Stem Cell Research at the University of Edinburgh in 1990 as a group leader. In 1996, he was appointed Director of the Centre. He was appointed MRC Research Professor in 2003. He took up the post of Director of the Cambridge Stem Cell Institute in the autumn of 2006. Professor Smith’s expertise is in the field of stem cell biology and he has pioneered key advances in the field of Embryonic Stem (ES) Cell research. His research focuses on the molecular and cellular controls of embryonic and somatic stem cells, and on interconversion between pluripotent and tissue-restricted states. |
| 16:15 - 16:45 |
Human pluripotent stem cells: the new "patient"?
Derivation of many different cell types from human pluripotent stem cells (embryonic stem cells or HESCs and induced pluripotent stem cells or hiPS cells) is an area of growing interest both for potential cell therapy and as a platform for drug discovery and toxicity. Most particularly, the recent availability of methods to introduce specific disease mutations into human pluripotent stem cells and/or to derive these cells as hiPS cells by reprogramming from any patient of choice, are creating unprecedented opportunities to create disease models “ in a dish” and study ways to treat it or slow down its rate of development. Understanding the underlying developmental mechanisms that control differentiation of pluripotent cells to their derivatives and mimicking these in defined culture conditions in vitro is now essential for moving the field forward. Professor Mummery and colleagues have used these methods to produce isogenic pairs of hiPSC lines to compare diseased and corresponding control cardiomyocytes and vascular endothelial cells and identify disease related phenotypes and mechanisms. The use of isogenic pairs has proved crucial since variability between “healthy control” hiPSC lines is often greater than the difference between a diseased cells and its isogenic control. Drug responses of hESC-derived cardiomyocytes to a variety of cardiac and non-cardiac drugs were examined and shown that iPSC derived cardiomyocytes with mutations in ion channel genes can accurately predict changes in cardiac electrical properties and reveal drug sensitivities also observed in patients. 
Professor Christine Mummery, Leiden University Medical Center, The Netherlands

Professor Christine Mummery, Leiden University Medical Center, The NetherlandsChristine Mummery studied physics at the University of Nottingham, UK and has a PhD in Biophysics from the University of London. After positions and postdoc and tenured group leader at the Hubrecht Institute, she became professor at the UMC Utrecht in 2002. In 2008, she became Professor of Developmental Biology at Leiden University Medical Centre in the Netherlands and head of the Department of Anatomy and Embryology. Her research concerns heart development and the differentiation of pluripotent human stem cells into the cardiac and vascular lineages and using these cells as disease models, for safety pharmacology, drug discovery and future cardiac repair. She is a member of the Royal Netherlands Academy of Science (KNAW), and board member of the International Society of Stem Cell research (ISSCR), the KNAW and the Netherlands Medical Research Council (ZonMW). She was recently awarded the Hugo van de Poelgeest Prize for Animal Alternatives in research. She co-authored a popular book on stem cells “Stem Cells: scientific facts and Fiction” in 2011 (2nd edition 2014) and is editor in chief of the ISSCR journal Stem Cell Reports. |
Chair

Professor Johan Hyllner, Cell Therapy Catapult, UK

Professor Johan Hyllner, Cell Therapy Catapult, UK
Johan is the Chief Scientific Officer of the Cell Therapy Catapult. As CSO, he is responsible for external scientific relations, assessment of candidate projects and technical oversight of preclinical programmes. He is currently Professor (adj) in Engineering Biology at Linköping University, Sweden and has a PhD from the University of Göteborg. Prior to joining the Catapult, he occupied a range of management roles in the cell therapy industry, in which he has worked for over 15 years, at companies such as Cellectis, Cellartis, and Vitrolife. He has extensive transaction experience and has been involved in many major collaborative projects with industry and academia. Current focus is on translation of a wide variety of cell therapies into the clinic. The technologies are based on several human cell sources including immune cells, and pluripotent and adult stem cells and cover disease areas like cancer, liver failure and stroke.
| 09:00 - 09:30 |
Discoveries and execution of the first specialised cell therapy (autologous chondrocytes) in global use
Cartilage injuries and the subsequent osteoarthritis (OA) disease have been an enigma for doctors since the age of Hippocrates. OA is characterized by cartilage degradation, formation of osteophytes and subchondral sclerosis that leads to joint destruction and severe impairment of mobility. OA is one of the most common forms of musculo-skeletal disease throughout the world with over 80 million patient affected and nearly 1 million patients are hospitalized each year in the U.S alone with a cost estimated to $60 billion. Currently no early diagnostic marker is available and no disease modifying treatment exists except for autologous chondrocyte transplantation (ACT) originally developed by our group. These facts are still a challenge for today’s cartilage research and were the driving force behind the development of the autologous chondrocyte transplantation technique in Gothenburg Sweden in the 1980s. The interdisciplinary collaboration and events leading to the first transplantation and its successful spread worldwide will be discussed and the clinical long term outcome after ACT will be presented. Furthermore, the scientific knowledge in cartilage regeneration and stem cell fields has increased as reflected by the significant increment in publications in the field. The cellular focus in the transplantation technology has revealed new understanding of the degenerative OA disease where we now have implications that the disease is basically a cell regenerative dysregulation and not a consequence of wear and tear. Future aspects of the treatment including the use of matrices and potentially new cellular concepts will also be discussed. 
Professor Anders Lindahl, The Sahlgrenska Academy, University of Gothenburg, Sweden

Professor Anders Lindahl, The Sahlgrenska Academy, University of Gothenburg, SwedenAnders Lindahl MD, Ph.D. is professor in cartilage regeneration at the Institute of Biomedicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg Sweden. He is the medical director of the cell transplantation unit and leading a research group focused on cartilage regeneration and osteoarthritis. He received his Ph.D. degree in 1986 and did his post-doc at Harvard Medical School 1987–1988. His research on human chondrocytes commenced in 1985 and pioneerde together with Drs. Peterson and Brittberg the use of autologous chondrocyte transplantation (ACT) for the treatment of cartilage defects. The study was published in New England Journal of Medicine (331:889-895, 1994) were he was the corresponding author and the article is one of the most cited in orthopedics. Over 1700 patients have been treated with ACT with cells processed in his laboratory. Fifteen PhD students and 5 post-docs have been under his supervision. He has written over 130 articles in the field of chondrocyte regeneration and stem cells with over 9000 citations. |
|---|---|
| 09:40 - 10:10 |
Cellular transplantation of human islets or stem cell progenitors in the treatment of diabetes
Transplantation of islets into the portal vein of patients with Type 1 diabetes is a safe and highly effective therapy to eliminate hypoglycaemia, and stabilise glucose control. The downside is that the treatment relies on a scarce cell source (human organ donors), and lifetime immunosuppression is needed. The process of extracting islets in GMP facilities requires considerable expertise, and is not universally available. The original Edmonton Protocol series (NEJM 2000) led to high rates of one-year insulin independence in a small number of patients. Most returned to insulin therapy at low dose by 3 to 5 years. Recent further advances in clinical islet transplantation have been substantial, and now at least 6 centres report insulin independence rates of over 50% at 5 years with T-depletional immunosuppression combined with anti-inflammatory antibodies. A large Phase 3 trial in North America will likely lead to imminent Biological Licensure for islet transplantation in the US. Human embryonic stem cell derived progenitors proffer a potential limitless source of islet precursors for further differentiate in vivo. These may be transplanted within immunoisolating membranes to ‘shield’ against allo and autoimmune attack. This approach is currently undergoing early pilot safety and feasibility investigation as part of first-in-human clinical trials. Rigorous safety trials are underway to define and mitigate these risks. Thus, islet transplantation provides a glimpse of the future potential of cellular transplantation in diabetes. Successful embryonic stem cell derived progenitors will potentially open up the market exponentially, provided early trials demonstrate safety and efficacy. 
Professor James Shapiro, University of Alberta, Canada

Professor James Shapiro, University of Alberta, CanadaProfessor James Shapiro was born in Leeds, England, son of a family doctor. He studied Medicine in Newcastle and trained in Surgery in Bristol. He developed a longstanding interest in islet transplantation as a medical student. He has been on Faculty at the UofA since 1998. James led the team that developed and tested the “Edmonton Protocol” and was the lead author on their seminal NEJM paper in 2000. This protocol revolutionized the treatment for Type 1 Diabetes, as for the first time a series of patients were able to completely stop their life-sustaining insulin injections. He is currently leading a National Canadian project in ex vivo organ transplant repair (CNTRP), and has active clinical trials in Edmonton evaluating caspase inhibitors and new subcutaneous devices for islet transplantation. He is leading diabetes clinical trials in stem cell transplantation and regenerative medicine. James is the recipient of a Gold Medal in Surgery, a Governor-General’s Gold Medal, a prestigious Hunterian Professor of the Royal College of Surgeons of England, and is a Fellow of the Royal Society of Canada. He currently holds a prestigious Canada Research Chair in Transplantation Surgery and Regenerative Medicine. |
| 10:20 - 10:50 |
Treatment of Parkinson’s disease using cell transplantation
In Parkinson’s disease (PD), the main pathology underlying motor symptoms is a loss of nigrostriatal dopaminergic neurons. Clinical trials with intrastriatal transplantation of human fetal mesencephalic tissue have shown that the grafted dopaminergic neurons reinnervate the striatum, restore striatal dopamine release and, in some cases, induce major, long-lasting improvement of motor function. However, non-motor symtoms originating from degeneration outside striatum or in non-dopaminergic systems are not alleviated by intrastriatal implantation of dopaminergic neurons. Stem cells and reprogrammed cells could potentially be used to produce dopaminergic neurons for transplantation in PD patients. Recent studies demonstrate that dopaminergic neurons of the correct substantia nigra phenotype can be generated from human embryonic stem cells in large numbers and standardized preparations, and are soon ready for patient application. Also dopaminergic neurons derived from human induced pluripotent stem cells are being considered for clinical translation. Important challenges will be to demonstrate the potency (growth capacity and functional efficacy) and safety of the generated dopaminergic neurons in preclinical animal models. The dopaminergic neurons should then be tested, using optimal patient selection and cell preparation and transplantation procedures, in controlled clinical studies. 
Professor Olle Lindvall, Lund University, Sweden

Professor Olle Lindvall, Lund University, SwedenOlle Lindvall received his Ph.D. in 1974 and M.D. in 1978 and became Professor and Senior Consultant in clinical neurology at Lund University Hospital, Sweden, in 1992. He is Senior Professor since 2014. He was leading the clinical cell transplantation program for Parkinson’s patients at Lund University Hospital between 1983 and 2012. This program pioneered the use of neuronal replacement as a novel therapeutic strategy to restore function in the diseased human brain. Lindvall's experimental laboratory is working with transplantation of stem cells and reprogrammed cells, and neurogenesis from the brain’s own neural stem cells after various insults. Olle Lindvall has published around 500 articles, review articles and book chapters. In 2007-2008, he Co-chaired the International Society for Stem Cell Research Task Force for the Clinical Translation of Stem Cells. Lindvall has received numerous Prizes and Awards. Since 2010, he is the Chairman for the Class for Medical Sciences at the Royal Swedish Academy of Sciences. |
| 11:30 - 12:00 |
Pioneering industrialisation of cell based therapies

Professor Silviu Itescu, Mesoblast, Australia

Professor Silviu Itescu, Mesoblast, Australia |
Chair

Professor Chris Mason, UCL, UK

Professor Chris Mason, UCL, UK
Professor Chris Mason holds the Chair of Regenerative Medicine Bioprocessing in the Advanced Centre for Biochemical Engineering, University College London. His main focus is the clinical translation and commercialization of cell and gene therapies. He has a multidisciplinary track record, spanning therapeutics, medical devices and information technology, clinical medicine, bioprocessing, regulation, healthcare economics, reimbursement and business. His current responsibilities include; Senior Editor of the journal "Regenerative Medicine", Chair of the BioIndustry Association, Regenerative Medicine and Cell Therapy Industry Group, Founder and CEO of the London Regenerative Medicine Network, and Trustee of the UK Stem Cell Foundation. Professor Mason is on a number of national and international committees, working groups and advisory boards enabling the clinical translation and commercialization of cell and gene therapies including; the UK-Israel Science Council, UK Regenerative Medicine Expert Group, the Scientific Advisory Panel of the UK Cell Therapy Catapult, and the Strategic Advisory Board of the Canadian Centre for the Commercialization of Regenerative Medicine. Professor Mason is a general spokesperson for the cell and gene therapy sector including frequent newspaper, radio and TV interviews plus social media including @Prof_ChrisMason on Twitter.
| 13:15 - 13:45 |
Stem cell approaches to treat cardiovascular diseases
Stem cell-based therapy is currently tested in several trials of both acute myocardial infarction and chronic heart failure. While the striking efficacy of early revascularization may question the rationale for an additional cell-based therapy in patients with an acute infarction, the situation is dramatically different for chronic heart failure when patients, the number of whom is escalating, have exhausted conventional treatments and are not candidates for more invasive procedures like cardiac transplantation or implantation of a mechanical assist device. The main question then is to determine how experimental data can be translated into clinical practice. To meet this objective, it is critical to more thoroughly decipher the mechanism of action of the transplanted cells and, more specifically, to validate the currently prevailing hypothesis that these cells fail to rebuild a myocardial tissue by themselves but rather act by harnessing endogenous repair pathways. Namely, the confirmation of this mechanism would have three major clinically relevant consequences: (1) the choice of the optimal cell type, based of head-to-head comparisons of the functional effects of secretomes derived from different cell types, although the already available comparisons clearly favor the use of cardiac-committed cells; (2) the optimization of early cell retention and survival, rather than of sustained cell engraftment, so that the cells reside in the target tissue long enough to deliver the factors underpinning their action, and (3) the reliance on banked, fully qualified allogeneic cells, the expected rejection of which should only have to be delayed since a permanent engraftment would no longer be the objective. One step further, the long term objective of cell therapy could be to use the cells as biofactories exclusively exploited for producing factors and then to only administer them to the patient along with controlled release delivery systems. The whole production process, including manufacturing, quality controls, regulation and costs, would then be closer to that of a biological pharmaceutic, thereby raising the hope of a facilitated and thus expended clinical use. 
Professor Philippe Menasche, Department of Cardiovascular Surgery, Hôpital Européen Georges Pompidou, Paris

Professor Philippe Menasche, Department of Cardiovascular Surgery, Hôpital Européen Georges Pompidou, ParisDr Philippe Menasché is currently Professor of Thoracic and Cardiovascular Surgery at the University of Paris Descartes, Chief of the Heart Failure Surgery Unit of the Hôpital Européen Georges Pompidou and Co-director of an INSERM (National Institute of Health and Medical Research) team devoted to cell therapy of cardiovascular diseases. He started to work in this area more than 15 years ago and performed in 2000 the first human intramyocardial transplantation of autologous skeletal myoblasts in a patient with severe heart failure. He was then the PI of a randomised controlled trial of myoblast transplantation designed to assess the safety and efficacy of the technique. The suboptimal results of this study have then led the group to refocus on the use of cardiac progenitors derived from human embryonic stem cells (ESC) for the treatment of severe heart failure and a clinical trial using these cells is currently starting. The group also works on tissue engineering for the treatment of chronic limb ischemia and replacement of the right heart outflow tract in children with congenital heart diseases. The laboratory is using small and large animal (including nonhuman primate) models of myocardial infarction and, in parallel with basic and preclinical studies on myocardial regeneration by ESC-derived cardiac progenitors, is involved in the optimisation of cell delivery and the assessment of cell biomimetics with the objective of a clinical translation in patients with end-stage heart failure who have exhausted conventional therapies. |
|---|---|
| 13:55 - 14:25 |
Fine-tuned T cell receptors for cancer immunotherapy
Human tumours are known to express unique antigens; however tumour immune evasion mechanisms often prevent effective naturally occurring anti-tumour immune responses. Adoptive T cell therapy, in which T Cell Receptors are engineered to identify cancer tumour antigens with increased affinity, is emerging as a promising strategy for the treatment of many forms of cancer, including those with historically bleak outcomes. Dr Jakobsen and colleagues have developed methods to engineer naturally occurring TCRs and enhance their ability to target and bind to cancer peptides thereby enabling a highly targeted immunotherapy. Unlike current antibody based therapies, affinity enhanced TCRs are able to target a larger pool of intracellular antigens presented on the cell surface as short peptides bound to human leukocyte antigen (HLA). This capability significantly increases the breath of targets, particularly as intracellular targets are known to be more closely associated with cancer. Target identification and validation, together with a broad and robust preclinical safety testing strategy are critical in the development of affinity-enhanced TCRs. Engineering TCRs requires balancing the need for higher affinity to the target peptide with the risk of cross-reactivity, which increases at higher affinities. Safety considerations include both on-target (antigens expression in normal tissues in addition to tumour) and off-tumour toxicity (recognition of other antigens). NY-ESO-1 is a cancer antigen which is expressed at high frequency (>30%) in a range of cancers including ovarian, prostate, NSCLC, myeloma, bladder, melanoma, oesophageal and breast. Affinity-enhanced TCRs have been developed to target NY-ESO-1. T cells transduced with affinity enhanced TCRs to NY-ESO are currently in clinical trials for synovial sarcoma, multiple myeloma, melanoma, ovarian and oesophageal cancers. Clinical data, to date, demonstrate encouraging rates of clinical responses and an acceptable tolerability profile. 
Dr Bent Jakobsen, Adaptimmune Limited, UK

Dr Bent Jakobsen, Adaptimmune Limited, UKDr Bent Jakobsen was co-founder of Avidex, the fore-runner of both Adaptimmune Ltd and Immunocore, two clinical stage biopharmaceutical companies focused on the use of T cell therapy to treat cancer. Prior to establishing these companies, Dr Jakobsen had a prestigious career in academia and headed up the Immune Receptor Group at the Institute of Molecular Medicine at the University of Oxford from 1993 to July 2000. This group was recognised as one of the leading international laboratories in molecular immunology and is a world leader in recombinant immune receptor technology. Dr Jakobsen was also a Senior Research Fellow of the Danish Natural Research Council, Aarhus, Denmark. This followed three years as a Post-doctoral researcher at the Laboratory of Molecular Biology of the Medical Research Council in Cambridge. |
| 14:05 - 15:35 |
Use of genetically modified T-cells in treatment of cancer

Professor Carl June, University of Pennsylvania, USA

Professor Carl June, University of Pennsylvania, USACarl June is the Richard W. Vague Professor in Immunotherapy in the Department of Pathology and Laboratory Medicine. He is currently Director of Translational Research at the Abramson Cancer Center at the University of Pennsylvania, and is an Investigator of the Abramson Family Cancer Research Institute. He is a graduate of the Naval Academy in Annapolis, and Baylor College of Medicine in Houston, 1979. He had graduate training in Immunology and malaria with Dr. Paul-Henri Lambert at the World Health Organization, Geneva, Switzerland from 1978-79, and post-doctoral training in transplantation biology with Dr. E. Donnell Thomas and Dr. John Hansen at the Fred Hutchinson Cancer Research Center in Seattle from 1983 - 1986. He is board certified in Internal Medicine and Medical Oncology. He founded the Immune Cell Biology Program and was head of the Department of Immunology at the Naval Medical Research Institute from 1990 to 1995 before joining the faculty of the Perelman School of Medicine in 1999. He maintains a research laboratory that studies various mechanisms of lymphocyte activation that relate to immune tolerance and adoptive immunotherapy for cancer and chronic infection. He has published more than 300 manuscripts and is the recipient of numerous prizes and honors, including election to the Institute of Medicine and the American Academy of Arts and Sciences. |
| 15:45 - 16:15 |
Cell therapies as medicines
In oncology and in some genetic diseases it is now clear that genetically manipulated cells have the potential to provide long lasting treatment responses. There is also promise in many other disease areas and a growing confidence that advances in cell/gene therapies will provide an important new treatment option beyond small molecules and biopharmaceutical drugs. Unlike the traditional areas of drug discovery and development, most of the early discovery work, construction of the “medicine”, and the clinical experiments are taking place within academic settings. This is opening up a rapidly expanding range of opportunities, but also raises a challenge: how can these treatments be made available more widely? There are questions about production, quality control, short and long term safety, measurement of effect, patient selection and monitoring, regulatory requirements, and costs to the healthcare systems around the world. In this talk I will outline some of the cell and gene therapies that are being worked on at GSK (in rare diseases and cancer) and across industry and discuss the approaches being taken to turn cell treatments into viable therapeutic options that can be used globally. 
Sir Patrick Vallance FMedSci FRS, Government Chief Scientific Adviser and Head of Government Science and Engineering Profession, UK Government

Sir Patrick Vallance FMedSci FRS, Government Chief Scientific Adviser and Head of Government Science and Engineering Profession, UK GovernmentSir Patrick Vallance is the UK Government Chief Scientific Adviser, National Technology Adviser and Head of the Government Science and Engineering profession. Previously, Patrick worked at GSK where he started in 2006 as Head of Drug Discovery, before becoming Senior Vice President, Medicines Discovery and Development and subsequently President, R&D at GlaxoSmithKline until 2017. Prior to this, Patrick was a clinical academic, Professor of Medicine and led the Division of Medicine at UCL. He has over 20 years’ experience of basic and clinical research. His personal research was in the area of diseases of blood vessels and endothelial biology. |
