Links to external sources may no longer work as intended. The content may not represent the latest thinking in this area or the Society’s current position on the topic.
OBSTRACT: interpreting the abstraction of tissue regeneration and re-engineering of female pelvic floor disorders in clinical practice
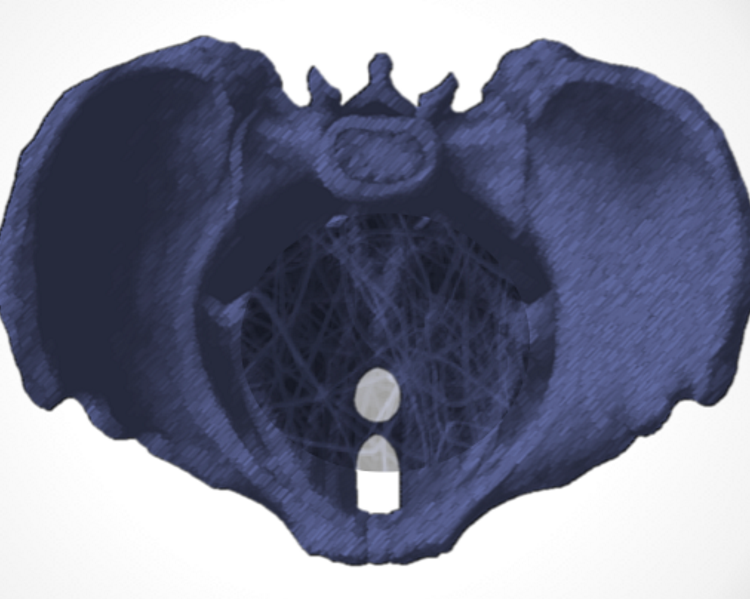
Scientific discussion meeting organised by Dr Sohier Elneil, Professor Sheila MacNeil, Professor Margot Damaser and Dr Gloria Esegbona.
This novel interdisciplinary meeting will bring together scientists and clinicians to review our current understanding of the pathophysiology of female pelvic floor disorders (PFD). Through an analysis of the molecular, cellular, biomechanical and neurological constituents that govern the trauma and subsequent healing process, and correlating this with the ambiguities plaguing the prognosis of surgical repairs. Investigate how our current technologies of regenerative medicine and tissue engineering may offer restorative properties of existing pelvic floor tissue. In addition, explore how these treatment methods can be used in a cost-effective, personalized and scientific approach to care.
The schedule of talks and speaker biographies will be available shortly. Recorded audio of the presentations will be available on this page after the meeting has taken place.
Enquiries: Contact the Scientific Programmes team.
Organisers
Schedule
Chair

Professor Margot Damaser, Cleveland Clinic Foundation

Professor Margot Damaser, Cleveland Clinic Foundation
Dr Damaser received her PhD in Bioengineering from the joint program of the University of California San Francisco and Berkeley. She is currently Professor of Molecular Medicine in the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and has joint appointments in the Biomedical Engineering Department and the Glickman Urological and Kidney Institute at Cleveland Clinic. She also is a Senior Research Career Scientist in the Advanced Platform Technology Center of Excellence of the Louis Stokes Cleveland VA Medical Center, Cleveland, OH. She has conducted research on the causes of and treatments for female pelvic floor disorders for over 20 years. She has developed and used animal models to test novel therapies with a focus on applying techniques from regenerative medicine to pelvic floor disorders. Dr Damaser has over 130 scientific peer-reviewed publications, has a number of patents pending, and has had research grants from NIH, US Dept of Veterans Affairs, private foundations, and several companies.
| 09:05 - 09:20 |
Why regenerative medicine for female PFD: rising incidence and challenges with current management
![LONDON, ENGLAND - MARCH 03: Dr Suzi Elneil photographed in the Charlotte Street Hotel on March 3, 2009 in London. (Photo by Bruno Vincent/Bruno -fVincent)-for further information contact Jane@dsapr.co.uk tel + 44 [0] 207553 3700](https://apidyn.royalsociety.org/Images/Events/People/316117B1-88A9-4365-82F1-F8B23447F0F6.jpg)
Dr Sohier Elneil, University College Hospital
![LONDON, ENGLAND - MARCH 03: Dr Suzi Elneil photographed in the Charlotte Street Hotel on March 3, 2009 in London. (Photo by Bruno Vincent/Bruno -fVincent)-for further information contact Jane@dsapr.co.uk tel + 44 [0] 207553 3700](https://apidyn.royalsociety.org/Images/Events/People/316117B1-88A9-4365-82F1-F8B23447F0F6.jpg)
Dr Sohier Elneil, University College HospitalSohier Elneil is a Consultant in Urogynaecology and Uro-neurology at University College Hospital and the National Hospital for Neurology and Neurosurgery in London. She started her medical career in Africa, where her focus was on capacity building in health service provision for women and girls suffering with genito-urinary tract fistulas and female genital mutilation. Both fields were, and continue to be, severely under-resourced in financial and manpower infrastructure. It was whilst carrying out this work, that she made a decision to pursue a career in these fields. The latter led her to pursue a doctorate at the University of Cambridge to study the physiology and pharmacology of sensory bladder dysfunction in women, following pelvic floor trauma, with a view to developing innovative therapies in the field. This formed the basis of her current research work in chemo- and electrical neuromodulation. Sohier Elneil is the Training Programme Director for Urogynaecology at University College London, and examines postgraduate students in Urogynaecology throughout the United Kingdom on behalf of the Royal College of Obstetricians and Gynaecologists, as well as in parts of Europe and Africa. She is a well-known national and international speaker in various fields including Urogynaecology, Uro-neurology, Pain Medicine, Global Maternal Health and other public health arenas. Sohier Elneil has multiple professional affiliations which are in keeping with all her academic and clinical achievements. They include President of the Royal Society of Medicine Pain Council, Chair of the International Continence Society (ICS) Fistula Committee, Chair of the Specialist Interest Group in Neuro-urogynaecology (International Urogynaecology Association) and now Chair of the RCOG Global Health Programs Committee. She also sits and Chairs several Charity Boards, amongst them being the Chair of the Board of Trustees of the Foundation for Women's Health Research and Development (FORWARD), since 2010. This is the leading African diaspora women’s campaign and support organisation, based in the United Kingdom that focuses on female genital mutilation (FGM), child marriage and obstetric fistula. It has achieved societal, legal and community change through its work with partnerships in the UK, Europe and Africa. |
|
|---|---|---|
| 09:20 - 09:40 |
Signalling away from scarring to regeneration

Dr Gloria Esegbona, King's College London

Dr Gloria Esegbona, King's College LondonDr Esegbona is an obstetrician and gynaecologist, scientist and Winston Churchill fellow with specialist experience in prevention and management of a wide range of trauma to the female pelvic tissues. She believes that there is a need to bring clarity to the abstract way in which the cellular and functional principles of the obstetric tract (OBSTRACT) is perceived and considered in clinical practice. A greater appreciation of this will improve clinical care - reducing the failure of surgical interventions and preventing the trauma in the first place during childbirth. |
|
| 09:40 - 10:15 |
Why regenerate? Morphological adaptations in pelvic tissue architecture, and extracellular matrix during birth
Pelvic floor muscles (PFMs) are integral for the proper function of the female pelvic floor. Epidemiological studies identify vaginal childbirth as a key risk factor for PFM injury and consequent dysfunction, which are critical for the development of pelvic floor disorders. Despite this, preventative strategies for maternal birth trauma continue to be non-existent and the available treatments offer marginal promise at best. Significant constraints, associated with directly probing PFMs in women, hinder the progress. We used an animal model, validated through a comparative analysis of the human and rat PFM architecture, to answer the following fundamental questions: (1) what are the effects of pregnancy on the contractile and extracellular matrix (ECM) muscle components? and (2) what are the mechanisms of pelvic muscles’ birth injury and subsequent dysfunction? Using rat model, we discovered a number of antepartum changes in the pelvic floor muscles, including fiber elongation via serial addition of sarcomeres, or sarcomerogenesis, and changes in ECM content and muscle stiffness. The results of our direct muscle properties’ measures of the PFMs, subjected to simulated birth injury in the absence and presence of pregnancy-induced adaptations will be discussed. Our findings provide a scientific rationale for the development of novel strategies, aimed at maximizing protective adaptations and regeneration of PFMs following birth injury. 
Professor Marianna Alperin, UC San Diego Health

Professor Marianna Alperin, UC San Diego HealthDr Alperin is a physician-scientist, whose expertise as a practicing female pelvic surgeon places her in a unique position, in which her clinical and surgical understanding help guide the direction of her basic science and translational studies to answer the most relevant clinical questions. Dr Alperin’s main research focus lies at the interface of female pelvic medicine and skeletal muscle physiology. To this effect, her studies concentrate on the urethral and anal sphincteric and pelvic floor muscles. Dr Alperin’s laboratory discovered the existence of pregnancy-induced adaptations in the pelvic muscles and has been actively identifying mechanisms that govern antepartum muscle plasticity. Her investigations also focus on the impact of vaginal delivery and aging, the leading risk factors for pelvic muscle dysfunction and related pelvic floor disorders, on the contractile, extracellular matrix, and cellular muscle components. |
|
| 10:15 - 10:40 | Scientific translation discussion | |
| 11:00 - 11:35 |
What are we regenerating? On the functional anatomy of the female pelvic floor
The goal of this presentation is to survey what is known and, more importantly, what is not known about how the pelvic floor works because knowledge gaps can hinder the selection of the most appropriate targets for tissue engineering to restore pelvic floor function. For example, the urethral sphincter is comprised of the urethral lumen, vascular plexus, longitudinal smooth muscle, circular smooth muscle, and circumferentially-oriented striated muscle. It is presently thought that the vascular plexus, smooth muscle, and striated muscle each contribute one-third of the urethral closure pressure required to maintain continence. But this dogma is based on one small study - hardly the quality of evidence upon which to decide which urethral layer needs to be regenerated in order to restore continence in an incontinent patient. Another example of a knowledge gap pertains to pelvic organ prolapse. Older dogma was that prolapse is caused by a defective vaginal wall. But newer research points instead to defects in apical and paravaginal tissues (which can be surgically corrected), as well as torn pubovisceral muscle. However a knowledge gap persists in the causes of prolapse in the absence of such defects; indications are that the re-innervation and regeneration of denervated muscle may be required. 
Professor James Ashton-Miller, University of Michigan

Professor James Ashton-Miller, University of MichiganAlbert Schultz Collegiate Research Professor and Distinguished Research Scientist, Departments of Mechanical Engineering, Biomedical Engineering and Internal Medicine, Institute of Gerontology, and the School of Kinesiology, Associate Vice President for Research, University of Michigan, U.S.A. |
|
| 11:35 - 12:10 |
Corpus intrapelvinum with intrapelvic urogenitodigestive diaphragm as the stabilising organ in functional pelvis anatomy and decisive factor in reconstructive pelvis surgery in the female
During extensive obstetric trauma surgery, many of the operation techniques have nothing to do with reconstructive pelvis surgery but more with financial profit to the medical industry. Other concepts are needed in order to understand the functional pelvis anatomy in the female, to identify the underlying defects and then to reconstruct the pelvis anatomy correctly using available autologous structures ensuring normal physiology and functioning. The different pelvis organs with their vascular supply and innervation are embedded into, protected by, and encapsulated or ensheathened by a complex connective tissue body/organ of pelvis as corpus intrapelvinum as part of the tela urogenitalis securing/ stabilising the organs in their variable anatomic position. This ensures their physiologic functioning independently or combined. The corpus intrapelvinum forms a 3-dimensional functional dymamic organ consisting of a cohesive mixture of collagen for strength, elastin for passive elasticity and plasticity and smooth muscle fibers for dynamic action in a loose, dense or condensed form all under control by the autonomic nervous system. One of its highly specialised structures is the intrapelvic urogenitodigestive diaphragm which supports the continence mechanisms and if intact prevents the high-pressure organs of the urinary tract, genital tract and digestive tract to prolapse into the zero-pressure vagina and then if not corrected further thru the vagina to the outside. Only by further research into the corpus intrapelvinum as a whole and into its highly specialised structures will it be possible to develop real reconstructive operation techniques ensuring normal physiology. 
Dr Kees Waaldijk, Babbar Ruga National Obstetric Fistula Centre

Dr Kees Waaldijk, Babbar Ruga National Obstetric Fistula CentreDr Waaldijk is a reconstructive obstetric trauma surgeon. He was born 1942 in Amsterdam, Holland, went to primary/secondary schooling and medicine study up till 1969 in Amsterdam. From 1969-70 he was an army doctor, and was then a resident training surgeon in traumatology and obstetrics/gynecology in Amsterdam 1970-1972. In 1971 he studied Tropical Medicine in Amsterdam, and then trained in leprosy in alert Addis Ababa in Ethiopia. Dr Waaldijk was a Medical Officer of health with special leprosy/tuberculosis control project Kwale district in Kenya 1972-1975, and trained in obstetrics/gynecology 1975 in Nijmegen, Holland. Further residency training surgery/traumatology 1975-76 in Goch, Germany. He became a Senior Consultant Surgeon in Kevelaer, Rees and Goch in Germany 1975-1983, and was a Senior Consultant in war surgery in Cambodian refugee camp Khao-I-Dang in Thailand. He became a Reconstructive Leprosy Surgeon 1983 in Addis Ababa in Ethiopia, Senior Consultant leprosy/tuberculosis Katsina State Nigeria 1983-2000. Today Dr Waaldijk is the Chief Consultant Obstetric Trauma Reconstructive Surgeon in Katsina, with some 25,000 obstetric trauma procedures, training of over 400 doctors and with many scientific publications and congress presentations. |
|
| 12:10 - 12:30 | Scientific translation discussion |
Chair
![LONDON, ENGLAND - MARCH 03: Dr Suzi Elneil photographed in the Charlotte Street Hotel on March 3, 2009 in London. (Photo by Bruno Vincent/Bruno -fVincent)-for further information contact Jane@dsapr.co.uk tel + 44 [0] 207553 3700](https://apidyn.royalsociety.org/Images/Events/People/316117B1-88A9-4365-82F1-F8B23447F0F6.jpg)
Dr Sohier Elneil, University College Hospital
![LONDON, ENGLAND - MARCH 03: Dr Suzi Elneil photographed in the Charlotte Street Hotel on March 3, 2009 in London. (Photo by Bruno Vincent/Bruno -fVincent)-for further information contact Jane@dsapr.co.uk tel + 44 [0] 207553 3700](https://apidyn.royalsociety.org/Images/Events/People/316117B1-88A9-4365-82F1-F8B23447F0F6.jpg)
Dr Sohier Elneil, University College Hospital
Sohier Elneil is a Consultant in Urogynaecology and Uro-neurology at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
She started her medical career in Africa, where her focus was on capacity building in health service provision for women and girls suffering with genito-urinary tract fistulas and female genital mutilation. Both fields were, and continue to be, severely under-resourced in financial and manpower infrastructure. It was whilst carrying out this work, that she made a decision to pursue a career in these fields. The latter led her to pursue a doctorate at the University of Cambridge to study the physiology and pharmacology of sensory bladder dysfunction in women, following pelvic floor trauma, with a view to developing innovative therapies in the field. This formed the basis of her current research work in chemo- and electrical neuromodulation.
Sohier Elneil is the Training Programme Director for Urogynaecology at University College London, and examines postgraduate students in Urogynaecology throughout the United Kingdom on behalf of the Royal College of Obstetricians and Gynaecologists, as well as in parts of Europe and Africa. She is a well-known national and international speaker in various fields including Urogynaecology, Uro-neurology, Pain Medicine, Global Maternal Health and other public health arenas.
Sohier Elneil has multiple professional affiliations which are in keeping with all her academic and clinical achievements. They include President of the Royal Society of Medicine Pain Council, Chair of the International Continence Society (ICS) Fistula Committee, Chair of the Specialist Interest Group in Neuro-urogynaecology (International Urogynaecology Association) and now Chair of the RCOG Global Health Programs Committee. She also sits and Chairs several Charity Boards, amongst them being the Chair of the Board of Trustees of the Foundation for Women's Health Research and Development (FORWARD), since 2010. This is the leading African diaspora women’s campaign and support organisation, based in the United Kingdom that focuses on female genital mutilation (FGM), child marriage and obstetric fistula. It has achieved societal, legal and community change through its work with partnerships in the UK, Europe and Africa.
| 13:30 - 14:05 |
A regenerative medicine approach to pelvic floor reconstruction: the host response as a primary determinant of success
Regenerative medicine approaches to soft tissue repair and reconstruction, including pelvic floor repair, typically involve the use of cells, scaffold materials, and/or bioactive molecules. Cell-based therapies, including the use of stem/progenitor cells, have largely failed to achieve successful clinical translation. However, biomaterial-based approaches, especially those that consist of naturally occurring materials, have had a significant positive influence upon the reconstruction of soft tissue structures such as the abdominal wall and skeletal muscle. The outcomes for any given clinical application depend upon several factors including biomaterial design, anatomic location, patient-specific factors, and surgical technique, among others. All implantable biomaterials elicit a host response, the nature of which logically influences the clinical outcome. Recent findings have shown that naturally occurring biomaterials can stimulate a constructive, immunomodulatory microenvironment and promote the recruitment, proliferation, and differentiation of stem cells into site-appropriate functional tissue. The relationship between the host response to biomaterials and clinical outcomes will be discussed, including the use of inductive immunomodulatory biomaterials for reconstruction of pelvic floor soft tissues. 
Professor Stephen F. Badylak, University of Pittsburgh

Professor Stephen F. Badylak, University of PittsburghDr Stephen Badylak, DVM, PhD, MD is a Professor in the Department of Surgery, and deputy director of the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. Dr Badylak holds over 60 U.S. patents, 200 patents worldwide, has authored more than 340 scientific publications and 40 book chapters, and has edited a textbook entitled “Host Response to Biomaterials”. He has served as the Chair of several study sections at the National Institutes of Health (NIH), and is currently a member of the College of Scientific Reviewers for NIH. More than eight million patients have been treated with bioscaffolds developed in Dr. Badylak’s laboratory.
|
|
|---|---|---|
| 14:05 - 14:40 |
Biomaterials and tissue engineering for pelvic floor regeneration
Age is unkind to women - childbearing and vaginal childbirth often lead to weakened pelvic floors and stress urinary incontinence (SUI) and/or pelvic organ prolapse (POP). 20% of healthy women will require surgery for POP by the age of 80. Non-degradable polypropylene (PP) meshes which have been used to support the pelvic organs for the last decade are now known to cause unacceptable side-effects in around 5% of woman when used as small tapes to support the urethra for SUI, and around 20% of woman when used as larger areas to support the pelvic organs in POP. Our team of scientists and clinicians are engaged in developing alternative next-generation biomaterials and tissue engineering approaches to provide solutions specifically designed for the dynamic pelvic floor. For SUI we have developed a fascia mimetic nondegradable mesh of polyurethane which has strength and elasticity much closer to the patient,s native tissue than the inflexible PP meshes currently used. This is currently being evaluated in a sheep vagina model developed by Professor Jan Deprest in Leuven.

Dr Sheila MacNeil, University of Sheffield

Dr Sheila MacNeil, University of SheffieldSheila is Professor of Tissue Engineering at Sheffield University ( non-clinical) with expertise in tissue engineering of skin, oral mucosa, urethra, oesophagus and cornea with a strong focus on translating research to the clinic. She has published over 350 peer-reviewed articles with over 7000 citations and has an h-index of 45. Since 1992 she has worked with clinicians in Sheffield, delivering autologous keratinocytes to burns patients and developed and commercialised improved delivery of skin cells to patients with MySkin™ and Cryoskin™ (autologous and allogeneic cell therapy for wound healing), available for NHS patients via Regenerys Ltd (www.regenerys.com). She has also worked extensively with NHS Urologist Professor Chris Chapple in Sheffield to develop tissue engineered oral mucosa for reconstruction of urethral stricture and published a 9 year follow-up on this recently. Another key project is developing cell delivery membranes for corneal defects (Wellcome Trust Affordable Healthcare for India) working with colleagues in India. She has also been developing an alternative material for support of the urethra with Professor Chris Chapple over the last 6 years which will better withstand the dynamic pressures in the pelvic floor. She received the UK Society of Biomaterials President’s medal for her contributions to Biomaterials in both the UK and overseas in September 2014. |
|
| 14:40 - 15:10 | Clinical translational discussion | |
| 15:30 - 16:05 |
Towards rebuilding vaginal support utilising extracellular matrix bioscaffolds

Professor Pamela Moalli

Professor Pamela MoalliDr Moalli is currently an Associate Professor in the Department of Obstetrics, Gynecology and Reproductive Sciences at the University of Pittsburgh with secondary appointments in the Department of Bioengineering, and the McGowan Institute of Regenerative Medicine. She is a senior scientist at the Magee-Womens Research Institute. After completing her undergraduate degree at Brown University, Dr Moalli received a combined MDPhD from Northwestern University. She served her residency in Obstetrics and Gynecology at Magee-Womens Hospital of the University of Pittsburgh and then completed a fellowship in Urogynecology and Reconstructive Pelvic Surgery at the same institution. Dr Moalli has been funded by the NIH since entering medical school in 1986. She progressed from the Medical Scientist Training Program to the Women’s Reproductive Health and Career Development Program (K 12) to become independently funded in several R01 grants and as a Co-Investigator on three U01 awards. She has over 96 peer reviewed publications and 9 book chapters. Her publications are equally divided between her laboratory work and her participation in multicenter trials. Dr Moalli is director of one of the only laboratories in the United States that employs a basic science approach to study mechanisms that contribute to the development of pelvic organ prolapse and the development of graft materials for use in Urogynecologic procedures. Over the last decade, her major research focus has been on understanding the pathogenesis of synthetic mesh complications which cause significant morbidity in affected women and have been the target of litigation world-wide. More recently, her group has been working on the development of novel regenerative materials that can be used to repair pelvic organ prolapse and urinary incontinence. Additional research interests include mechanisms of maternal birth injury and improving outcomes in women injured during childbirth. |
|
| 16:05 - 16:40 |
Biomechanics of female pelvic cavity: lessons for regeneration from computational models
Female pelvic floor dysfunction can result from weakening or damage of the pelvic floor muscles or connective tissue. It is studied in the clinical setting, and computational biomechanical analysis has contributed along the years, in a translational research basis. Computational frameworks to study the childbirth influence in the mechanical behavior of the pelvic floor will be presented. Besides the experience in characterizing and modeling the mechanical properties of soft tissues, there is a lack of tools to predict the requirements for implementation of regenerative solutions to the pelvic tissues. It has been observed that macro-scale biomechanical evidences of damage in tissue may be detected earlier at a micro and nano-scale of the extracellular matrix structure. Pelvic supportive tissues have high demands on functionality, however their material properties vary significantly interpersonally. The balance between synthesis and degradation of collagen on the ECM maintain the tissue integrity and tensile strength. Any deviation from the normal conditions will change the mechanotransduction processes and produce damage into the tissue. In the context of a mechanical approach to cell biology, there is a close relationship between cellular function and mechanical properties. Given the importance of the actin contraction on the physiological functions, the establishment of a constitutive model to describe how the filamentous network controls its mechanics actively is crucial. Assuming an enthalpic deformation of isotropic cross-linked network as the dominant contribution to elasticity, the development of a suitable constitutive model will be discussed. 
Professor Renato Jorge, University of Porto

Professor Renato Jorge, University of PortoRenato Natal Jorge is graduated in Mechanical Engineering from the University of Porto, Portugal, since 1987. In 1991, he obtained the Master degree in Structural Mechanics, also at University of Porto. In 1999, he obtained the PhD degree in Mechanical Engineering from the same University. |
|
| 16:40 - 17:00 | Clinical translational discussion |
Chair

Dr Pedro Martins, University of Porto

Dr Pedro Martins, University of Porto
Pedro Martins is a Postdoctoral researcher (INEGI, FEUP) and Invited Assistant Professor, in the department of Mechanical Engineering at the Faculty of Engineering of Porto University (FEUP, Portugal). Pedro completed his Ph.D. in Mechanical Engineering with application to biomechanics, at FEUP. His undergraduate studies, in theoretical physics, were developed at the Faculty of Sciences of Porto University (FCUP). His research has been focused on the mechanical properties of biological tissues and biomaterials. He has studied the female pelvic floor tissues, under normal and pathological conditions. He has collaborated with several researchers, on multidisciplinary research projects, on domains that range from applied mechanics to image processing. He his author and co-author of more than 40 research papers, was awarded 3 individual merit fellowships and has is the PI of 3 research projects in the field of Biomechanics.
| 09:00 - 09:35 |
Potential for biomaterials and bioactive signal expression in engineered materials for pelvic floor disorders
Thousands of surgical procedures are performed every day. Tissue is removed or damaged for different reasons. In the past these wounds healed with scar tissue. This scar tissue is a natural part of the healing process, however, it does not have the same flexibility and elasticity as healthy/original muscle. This presentation will summarise the current research results that will define new healing treatments but also address improvements to optimise outcomes for those patients with pelvic floor disorders. 
Professor Karl-Dietrich Sievert, University Clinic of Rostock

Professor Karl-Dietrich Sievert, University Clinic of RostockProfessor Karl-Dietrich Sievert, MD, PhD, FACS, FRCS, was the Chair of Urology and Andrology, Director of Uro-oncology, Neuro-urology, Incontinence and Reconstructive Urology at Salk Clinic and Parcelsus Medical University (PMU) in Salzburg, Austria and Director of the Department of Urology at the University of Lubeck (UKSH). Previously he was the Vice Chair of Urology for more than 10 years at the University of Tübingen. Professor Sievert has participated in visiting professorships and held international training courses, published in the fields of oncology, neuro-urology, incontinence, and reconstructive surgery and is a board member of numerous urological and reconstructive surgery organizations. He has received funding and awards for his research and is a recurring meeting contributor for both his clinical and basic research work to the local and international community, such as the European, German and American Urology Associations, International Continence Society, and Global Congress on Lower Urinary Tract Dysfunction, including State-of-the-Art presentations in oncology, incontinence, tissue engineering and stem cell treatments. In both 2011 and 2013, he was elected as Chairman of the ESFFU-ERGURS meeting and the 2012 AuF, Germany’s Urology Research meeting. In 2016, he was elected as Co-Chairman of the Global Congress on Lower Urinary Tracy dysfunction meeting in Vienna, Austria.
|
|
|---|---|---|
| 09:35 - 10:05 |
Characteristics of vaginal fibroblasts, their attachment to biomaterials and potential application of cell-based therapy for treatment of female Pelvic Floor Disorders
Currently Pelvic Floor Disorders (PFDs) affect almost half of post-menopausal women. The frequency and severity of symptoms increase after menopause, which may be due to loss of protective effects of ovarian hormones leading to faster deterioration of the pelvic floor extracellular matrix. Thus, we studied fundamental molecular mechanisms underlying the development of Pelvic Organ Prolapse (POP) in pre- and post-menopausal women using molecular biological, biochemical, and immunohistochemical approaches. Our focus was on in vivo expression of genes and proteins participating in elastogenesis and collagenogenesis, as well as major proteolytic enzymes and their tissue inhibitors. The effect of Local Estrogen Therapy was assessed in vaginal biopsies of post-menopausal women with severe POP. We observed an increase in the expression of structural proteins (collagen/elastin) and local activation of the immune system, which supports a beneficial role of Estrogen in the remediation of pelvic floor tissue in POP patients. 
Assistant Professor Oksana Shynlova, University of Toronto

Assistant Professor Oksana Shynlova, University of TorontoDr Shynlova has a PhD in Biochemistry from Kiev, Ukraine. Dr Shynlova’s research in Lunenfeld-Tanenbaum Research Institute at Sinai Health System, Toronto, Canada focuses on several areas of interest, including studies of fundamental molecular mechanisms underlying the development of Pelvic Floor Disorders (PFDs), in particular Pelvic Organ Prolapse (POP) in pre- and post-menopausal women. Using molecular biological, biochemical and immunohistochemical approaches, she examined the extracellular matrix in vaginal tissue of women with advanced POP. The focus was on in vivo expression of genes and proteins participating in elastogenesis and collagenogenesis, as well as the expression of major proteolytic enzymes and their tissue inhibitors. Dr Shynlova developed a methodology that characterized autologous vaginal cells isolated from the tissue of patients with POP. Her current interest is in generating autologous muscle cells and fibroblasts from urine-derived somatic cells reprogrammed to Pluripotent Stem Cells in attempt to revolutionize modern urogynecological treatment of PFDs. |
|
| 10:05 - 10:40 | Implantation strategy discussion | |
| 11:00 - 11:35 |
Tissue engineering approaches for treating Pelvic Organ Prolapse (POP) using a novel source of mesenchymal stem/stromal cells and new materials
We are using a tissue engineering approach to address problems associated with using vaginal mesh for treating POP. We propose to use autologous endometrial mesenchymal stem cells (eMSC) delivered on polyamide/gelatin composite meshes (eMSC/PA+G) to improve mesh biocompatibility and regenerate vaginal tissue damaged from childbirth injury. eMSC are purified from biopsies obtained from premenopausal and short-term estrogen-treated post-menopausal women using SUSD2 magnetic-bead sorting. eMSC are culture-expanded in serum-free medium under hypoxia with a small molecule TGFβ-receptor inhibitor, A83-01, generating 90-95% SUSD2+ eMSC. ATACseq and RNAseq revealed that A83-01 maintains eMSC stemness by opening chromatin loci enriched for transcription factor binding sites and upregulating gene networks involved in developmental and stem cell signalling pathways. A rat model treated with human eMSC/PA+G constructs showed that eMSC increased vascularisation, reduced chronic inflammation, promoted deposition of crimped collagen, generating a biomechanically less stiff mesh/tissue complex compared to PA+G. The eMSC had a paracrine mechanism of action in improving biocompatibility of PA+G mesh. We are using an autologous preclinical ovine model of vaginal surgical repair in multiparous ewes. POP is assessed using a modified POP-Q and our novel fibre-optic pressure sensor device. Ovine eMSC are isolated from hysterectomies by FACS sorting CD271+CD49f cells, culture-expanded and labelled with IODEX-FITC paramagnetic nanoparticles for cell tracking before seeding on PA+G mesh and implanting transvaginally using urogynaecological surgical procedures. 
Associate Professor Caroline Gargett, Hudson Institute of Medical Research

Associate Professor Caroline Gargett, Hudson Institute of Medical ResearchAssociate Professor Caroline Gargett is a Senior Research Fellow of the National Health & Medical Research Council of Australia, Deputy Director of the Ritchie Centre and heads the Endometrial Stem Cell Biology Laboratory at the Hudson Institute of Medical Research. She has an adjunct appointment in the Department of Obstetrics and Gynaecology, Monash University. She discovered adult stem cells in the endometrium as well as specific markers to purify these epithelial progenitors and mesenchymal stem cells. She investigates their role in endometriosis and infertility and is developing endometrial mesenchymal stem cells as a cell-based therapy for gynaecological applications. She has received a number of international awards from ESHRE, Society for Reproductive Investigation and the Endometriosis Foundation of America. She is a Board Member of the National Stem Cell Foundation of Australia and on the Editorial Boards of Biology of Reproduction, Reproductive Sciences and Scientific Reports. |
|
| 11:35 - 12:10 |
Modulating the regeneration response: regulation of cellular microenvironments and novel solutions to translational hurdles
Engineering complex tissues creates major challenges for tissue engineering and regenerative medicine strategies. Complex form and function in tissues such as the pelvic floor comprise multiple musculoskeletal tissues with cross-tissue integration and junctions. Our lab has been involved in understanding the ways we can engineer a stem cell niche which can go on to regenerate multiple tissues. Our aim is to deliver donor progenitor cells which can build and define replacement tissues in vivo. The challenge lies in engineering control solutions for stem cells which have clinical relevance. We have developed multiple control systems which can be aligned with cell based therapies and have gone some way towards translating these approaches towards first in man. In this presentation I will describe our novel stem cell bandage which can be used for enhancing and maintaining stem cell communities in bone. I will present our work in nanomedicine with remote magnetic nanoparticle control strategies for stem cell activation and optimised differentiation which is now in preclinical trial. Finally I will align these strategies to potential treatments for pelvic floor disorders and consider how we can translate these approaches towards a clinical solution for patients 
Professor Alicia El Haj, Keele University

Professor Alicia El Haj, Keele UniversityProfessor Alicia El Haj is a leading UK figure in Bioengineering and Regenerative Medicine and has been involved in bringing together interdisciplinary groups within biomedicine, physical sciences and engineering to develop innovative new cell based therapies to the clinic. She has published over a 300 publications in novel enabling technologies such as novel cell and tissue engineering approaches , bioreactors technology, biomaterials and new imaging systems for regenerative medicine with funding from EPSRC, MRC, BBSRC, AR UK Innovate and EU FP in the UK. She is a co-Director of a CDT programme in Regen Med, and a UKRMP Hub in Engineering the Stem cell niche. ISTM has been progressing cell therapies into routine clinical use in orthopaedics for the past 10 years working with consultant orthopaedic surgeons. She is also a Director of a spin out company MICA Biosystems , Ltd involved in translating her patents into clinical use. Professor El Haj was awarded a Royal Society Merit Award in 2014 and in March 2015, was awarded the MRC Suffrage Award for her role in leading women in STEM . |
|
| 12:10 - 12:30 | Implantation strategy discussion |
Chair

Dr Sheila MacNeil, University of Sheffield

Dr Sheila MacNeil, University of Sheffield
Sheila is Professor of Tissue Engineering at Sheffield University ( non-clinical) with expertise in tissue engineering of skin, oral mucosa, urethra, oesophagus and cornea with a strong focus on translating research to the clinic. She has published over 350 peer-reviewed articles with over 7000 citations and has an h-index of 45. Since 1992 she has worked with clinicians in Sheffield, delivering autologous keratinocytes to burns patients and developed and commercialised improved delivery of skin cells to patients with MySkin™ and Cryoskin™ (autologous and allogeneic cell therapy for wound healing), available for NHS patients via Regenerys Ltd (www.regenerys.com). She has also worked extensively with NHS Urologist Professor Chris Chapple in Sheffield to develop tissue engineered oral mucosa for reconstruction of urethral stricture and published a 9 year follow-up on this recently. Another key project is developing cell delivery membranes for corneal defects (Wellcome Trust Affordable Healthcare for India) working with colleagues in India. She has also been developing an alternative material for support of the urethra with Professor Chris Chapple over the last 6 years which will better withstand the dynamic pressures in the pelvic floor.
She received the UK Society of Biomaterials President’s medal for her contributions to Biomaterials in both the UK and overseas in September 2014.
| 13:30 - 14:00 |
Non-cellular regenerative medicine techniques for prevention of female pelvic floor disorders
One of the greatest risk factors for development of pelvic floor disorders is vaginal delivery of children, which can damage the nerves and muscles responsible for continence in addition to the connective tissues responsible for the position of pelvic organs. Without complete nerve regeneration, the muscles can atrophy. Pelvic floor connective tissue can fail to restore the vascularisation necessary for full regeneration. Current treatments, such as an implanted mesh or sling, can have a high rate of complications and a high revision rate. Regenerative interventions done soon after delivery of children, prior to muscle atrophy, has the potential to have an increased success rate and to prevent pelvic floor disorders. Regenerative medicine usually involves local treatment with autologous stem or progenitor cells. This approach has had mixed results in clinical trials with women with stress incontinence. The mechanism of action of these cells is likely via their secretions. Therefore, a non-cellular approach with fewer risks could be to give the secretions of stem cells rather than inject the cells themselves. This approach also holds the promise of providing an off-the-shelf treatment. This talk will summarise our preclinical studies which demonstrate that treatment with secretions of cells provide a strong regenerative effect, even when given systemically. In addition, Professor Damaser will present preclinical data demonstrating that electrical stimulation could also promote neuro-regeneration and facilitate repair. Non-cellular therapies, such as these, given soon after childbirth in women at greatest risk of development of pelvic floor disorders hold great potential to improve the lives of many women. 
Professor Margot Damaser, Cleveland Clinic Foundation

Professor Margot Damaser, Cleveland Clinic FoundationDr Damaser received her PhD in Bioengineering from the joint program of the University of California San Francisco and Berkeley. She is currently Professor of Molecular Medicine in the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and has joint appointments in the Biomedical Engineering Department and the Glickman Urological and Kidney Institute at Cleveland Clinic. She also is a Senior Research Career Scientist in the Advanced Platform Technology Center of Excellence of the Louis Stokes Cleveland VA Medical Center, Cleveland, OH. She has conducted research on the causes of and treatments for female pelvic floor disorders for over 20 years. She has developed and used animal models to test novel therapies with a focus on applying techniques from regenerative medicine to pelvic floor disorders. Dr Damaser has over 130 scientific peer-reviewed publications, has a number of patents pending, and has had research grants from NIH, US Dept of Veterans Affairs, private foundations, and several companies. |
|
|---|---|---|
| 14:00 - 14:30 |
Preclinical animal models for pelvic organ prolapse
The cause of pelvic organ prolapse (POP) is multifactorial yet includes a complex interplay between genetic factors, vaginal birth-induced trauma, aging and lifestyle. Currently, surgery is the mainstay of therapy and the life-time risk is 19% by the age of 802. Given the relevance of the condition and the failure of current strategies a better understanding of the genesis of POP and the potential for its prevention or improving current therapies appropriate animal models are required. Finding an optimal model is challenging, since humans are bipedal, have no tail, and the foetal head is relatively large compared to the pelvic dimensions, making vaginal delivery more traumatic when compared to other species. This talk will discuss both pelvic organ prolapse and birth induced injuries in animal models 
Professor Jan Deprest, UZ Leuven

Professor Jan Deprest, UZ LeuvenJan Deprest MD PhD FRCOG is Professor of Obstetrics and Gynaecology at the Faculty of Medicine (KU Leuven) and its University Hospitals Leuven and at University College London. He is the Academic Chair (2012-2021) of the Department of Development and Regeneration, under which Obstetrics and Gynaecology is resorting. He is the Director of the Training Center for Surgical Technologies, with Prof Paul Herijgers. He is member of the Group Board of Biomedical Sciences KU Leuven (2014-2017). In Leuven, he is the clinical director of the fetal therapy program as well as the co-director of program for female pelvic floor dysfunction. He is also a member of the staff of Gynaecology. His research is mainly translational and dedicated to surgical treatment options as well as cell therapy solutions for (prevention of) pelvic floor dysfunction. His clinical research into urogynaecology is focused on the use of imaging, outcomes of implant surgery and surgical treatment of level I defects. He published over 470 peer reviewed papers and supervises/d over 25 doctoral students. He is Editor of Prenatal Diagnosis (Section: Fetal Imaging and Therapy), Editor in Chief Gynaecologic Surgery (2013), and International Editorial Board member of BJOG. He is board member of the national society of Obstetrics and Gynaecology (V.V.O.G) where he is chairing the scientific committee. He serves on the scientific committee of ESGE and EUGA |
|
| 14:30 - 15:00 | Discussion | |
| 15:20 - 15:45 |
Safety and performance of a wireless implantable tibial nerve stimulator device, for the treatment of patients with overactive bladder (OAB)
Overactive bladder (OAB) affects millions of people worldwide with neuromodulation offering treatment option for refractory patients. A novel peripheral implantable neurostimulator device for the treatment of OAB was developed (RENOVA iStim™ system), which electrically stimulates the tibial nerve at the site just proximally of the medial malleolus. The implant is wirelessly powered by a wearable unit that controls the therapeutic parameters and is worn by the patient during treatment at home. A Clinician Programmer is used to remotely set individual stimulation parameters. Herewith, the safety and performance of the RENOVA iStim™ system is being observed for the treatment of OAB. Short term; 6-mos results, and long term; 24-mos partial results, will be presented. 
Dr Guri Oron, BlueWind Medical

Dr Guri Oron, BlueWind MedicalDr Guri Oron has co-founded Bluewind and been with the company since its inception. He has over 15 years of experience in executive R&D roles, primarily with startups in the medical device field with specific expertise in Neurostimulation technologies. Throughout his career Dr Oron managed complex R&D projects from their ideation through design, engineering, regulatory and clinical development. Prior to working with BlueWind Medical, led the R&D at BrainsGate and before that in defence R&D roles. Dr Oron holds a BSc in Electrical Engineering from Tel Aviv University. He is the lead inventor or co-inventor of numerous patent and patent pending applications. |
|
| 15:45 - 16:10 |
Mechanical and etiological aspects of female pelvic floor disorders
It is commonly accepted that Pelvic floor disorders (PFD) have many risk factors. However, there is no consensus on their scope and importance. Some are specific for an individual (number of pregnancies) others common to a group or population. A complete and coherent map of prolapse etiology remains an open question. A new and promising frontier lies on the fusion of statistical/epidemiologic information with individual-level knowledge. The estimation of tissue’s mechanical behaviour based on patient specific data, may in turn, feed epidemiologic models with predictive capabilities. Such models bring an array of possibilities and implications - improved clinical diagnostics, PFD risk assessment, improved surgical outcome estimation, etc. To build such tool(s) will require a multidisciplinary effort, between medical doctors, engineers, and mathematicians. 
Dr Pedro Martins, University of Porto

Dr Pedro Martins, University of PortoPedro Martins is a Postdoctoral researcher (INEGI, FEUP) and Invited Assistant Professor, in the department of Mechanical Engineering at the Faculty of Engineering of Porto University (FEUP, Portugal). Pedro completed his Ph.D. in Mechanical Engineering with application to biomechanics, at FEUP. His undergraduate studies, in theoretical physics, were developed at the Faculty of Sciences of Porto University (FCUP). His research has been focused on the mechanical properties of biological tissues and biomaterials. He has studied the female pelvic floor tissues, under normal and pathological conditions. He has collaborated with several researchers, on multidisciplinary research projects, on domains that range from applied mechanics to image processing. He his author and co-author of more than 40 research papers, was awarded 3 individual merit fellowships and has is the PI of 3 research projects in the field of Biomechanics. |
|
| 16:10 - 16:40 |
Tissue engineering and regeneration: regulatory routes to the clinic
Developing a new medical product requires an understanding of the regulatory environment: how the law views the product and which agencies must be involved in the process. This talk will highlight the different ways in which regenerative medicine products can be regulated in the UK/EU, which in turn dictates how they can be brought into clinical use. Authorisation for clinical trials, processes and supporting data requirements are all entirely dependent upon how a TE/RM product is regulated: as a medical device, a human tissue product or a medicinal product / ATMP. Although perhaps not as exciting as the scientific and clinical challenges involved, an appreciation of the regulatory position early in the process can only increase your chances of successfully developing a safe, effective product that reaches patients in a timely way. 
Ms Alison Wilson, CellData Services

Ms Alison Wilson, CellData ServicesAlison Wilson is an independent regulatory affairs consultant with over 25 years’ experience in medicinal products, cell-based therapies and medical devices. She is a nominated UK expert for ISO (International Standards Organisation) TC150/WG 11 - Tissue Engineered Medical Products, and a member of the British Standards Institute Regenerative Medicine Technical Panel. Alison is a Module Advisor for the TOPRA MSc in Regulatory Affairs. She has contributed to several BSI cell therapy publications and has several publications on regulation of regenerative medicine products. Alison provides strategic regulatory and development advice for companies developing commercialise advanced therapy medicinal products (ATMPs) and regenerative medicine therapies in the EU. Alison is a Fellow of TOPRA and in 2014 received the first TOPRA Futures Award for contribution to regulatory science in new technology areas. |
|
| 16:40 - 17:00 | Discussion |
