Links to external sources may no longer work as intended. The content may not represent the latest thinking in this area or the Society’s current position on the topic.
Royal Society Africa Prize Seminar 2019
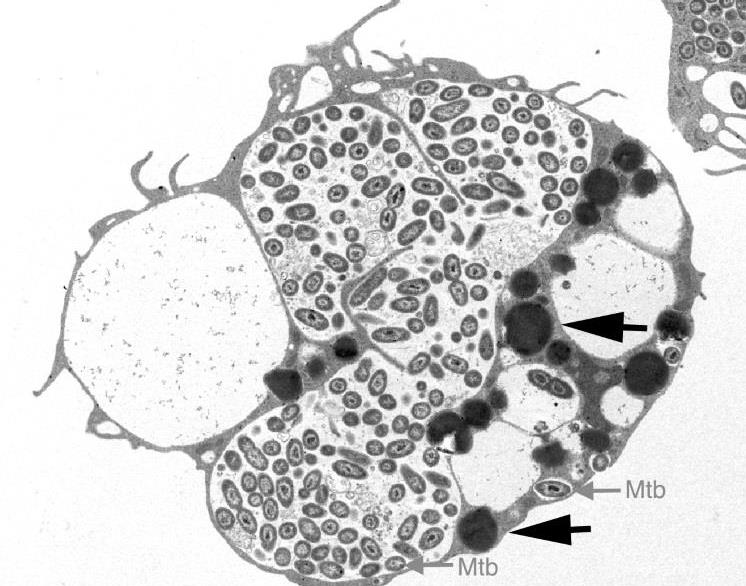
Royal Society Africa Prize Seminar 2019 with Dr Henry Mwandumba
The Royal Society Africa Prize recognises research scientists based in Africa who are making an innovative contribution to the biological sciences, including basic medical science, which contributes significantly to capacity building in Africa.
The Royal Society Africa Prize 2019 is awarded to Dr Henry Mwandumba for his novel work in description of the TB phagosome in HIV infected alveolar macrophages and his leadership in the College of Medicine in Malawi.
This afternoon seminar, held as part of the Prize, celebrated Dr Mwandumba’s work and explored related research. The seminar was chaired by Professor David Lalloo, Liverpool School of Tropical Medicine. The audio recording of the seminar talks and discussion is available on this page.
For all enquiries, please contact the Events team.
Schedule
Chair

Professor David Lalloo, Liverpool School of Tropical Medicine, UK

Professor David Lalloo, Liverpool School of Tropical Medicine, UK
Professor David Lalloo is Professor of Tropical Medicine and Director of the Liverpool School of Tropical Medicine. He also holds an Honorary Consultant position at the Royal Liverpool University Hospital. He trained in General Medicine, Infectious Diseases and Tropical Medicine, spending three years in Papua New Guinea and undertaking clinical and laboratory research in Oxford, before moving to the Liverpool School of Tropical Medicine in 1999. His main research interests are in epidemiological studies and clinical trials in resource poor settings, particularly in HIV related infections, malaria and envenoming. He currently has collaborations and studies in a number of countries including Malawi, Sri Lanka, Uganda, South Africa, Kenya, Eswatini, Nigeria and Vietnam. He also has a strong interest in guiding the careers of young UK and African scientists and supporting African research capacity strengthening. He chairs the NIHR Global Health Research Independent Scientific Advisory Group and is a member of the MRC Global Health Group.
| 15:35 - 16:05 |
Improving access by the poor to TB prevention, care and cure; the context for which we need new tools to end TB
This talk will outline briefly the Global Burden of tuberculosis (TB), the infectious disease that currently kills more people annually than HIV and malaria combined. It will go on to describe how TB is closely associated with poverty, both as a cause and consequence of the disease. Health systems globally, but particularly in Low & Middle Income Countries, are struggling to lower the substantial hurdles that people have to overcome in order to be diagnosed with TB, treated, cured and returned to good health. In these contexts, treatment of disease is currently the most effective means of preventing transmission and the establishment of new infections, but leaves a pool of individuals who already have latent infection untreated and who may progress to develop disease. This is the context for which we need new tools for prevention (vaccination and chemoprophylaxis), diagnosis (of latent and active disease), and treatment (of both latent and active disease). Dr Mwandumba’s work on the immune responses in the lung is making a substantial contribution to the understanding of the cellular mechanisms that form the discovery foundation for the generation of the new tools that are so desperately needed. 
Professor Bertie Squire, Liverpool School of Tropical Medicine, UK

Professor Bertie Squire, Liverpool School of Tropical Medicine, UKProfessor Bertie Squire studied medicine and immunology at University College London and Cambridge University before professional training in internal medicine, infectious diseases and respiratory medicine at the Royal London Hospital and the Royal Free Hospital. From 1992 to 1995 he was Head of the Department of Medicine, Kamuzu Central Hospital, Lilongwe, Malawi. Since 1995, Bertie has been primarily based at LSTM. He collaborates with many individuals and organisations in a programme of multi-disciplinary applied health research aimed at providing knowledge for action in making health services for tuberculosis more accessible to poor people in developing countries (particularly those affected by the HIV pandemic). He holds an appointment in the UK National Health Service as Honorary Consultant in Infectious Diseases and Tropical Medicine at the Royal Liverpool University Hospital and was President of the International Union Against Tuberculosis & Lung Disease from 2007 to 2011. |
|---|---|
| 16:05 - 16:35 |
Exploiting HIV infection to understand pathogen-specific immune responses in the lung
The lung is one of the organs most commonly affected by complications of human immunodeficiency virus (HIV) infection. While the use of potent antiretroviral therapy (ART) has led to a dramatic fall in the burden of respiratory diseases among HIV-infected individuals, the incidence of infections such as tuberculosis (TB) remains higher in HIV-infected adults receiving ART compared to HIV-uninfected individuals. This increased susceptibility is greatest during the first few years following ART initiation and may be due to persistent impairment of both innate and mycobacteria-specific adaptive pulmonary immune responses observed in HIV-infected ART-naive adults. Our work with HIV-infected adults in Malawi has shown that HIV-infected adults on ART have impaired alveolar macrophage function and reduced mycobacteria-specific alveolar CD4+ T-cell responses, most marked early during ART, and may partly explain the high risk of TB in these individuals. We have also reported persistence of HIV in airway macrophages and how HIV-infected macrophages can be targets as part of the effort to find a cure for HIV. These findings underscore the need for strategies to augment ART to improve lung immune cell function and reduce the high incidence of TB. The development and use of subunit vaccines that promote repopulation of the lung with crucial mycobacteria-specific polyfunctional CD4+ T-cell subsets and the use of prophylactic TB chemotherapy in HIV-infected adults, especially during the early years of ART when they are most vulnerable should be explored. 
Dr Henry Mwandumba, University of Malawi College of Medicine, Malawi, and Liverpool School of Tropical Medicine, UK

Dr Henry Mwandumba, University of Malawi College of Medicine, Malawi, and Liverpool School of Tropical Medicine, UKHenry Mwandumba is Professor of Medicine at the University of Malawi College of Medicine in Blantyre, Malawi, and at the Liverpool School of Tropical Medicine in Liverpool, UK. He is Honorary Consultant Physician at Queen Elizabeth Central Hospital in Blantyre and at the Royal Liverpool and Broadgreen University Hospitals NHS Trust in Liverpool. Henry is Deputy Director of the Malawi-Liverpool-Wellcome Trust Clinical Research Programme (MLW) and Head of the Mucosal and Vascular Immunology Group at MLW in Blantyre. He studied medicine at the University of Zimbabwe School of Medicine in Harare, Zimbabwe and specialised in General Internal Medicine and Infectious Diseases in Liverpool. Henry obtained a Diploma in Tropical Medicine and Hygiene from the Liverpool School of Tropical Medicine and for the last decade, he has been based at MLW where he conducts research focusing on understanding the effects of HIV-1 infection on lung immunity and predisposition to respiratory infections, especially tuberculosis. He is President of the Federation of African Immunological Societies, member of Council of the International Union of Immunological Societies, Immediate Past President of the Immunology Society of Malawi and Treasurer of the East, Central and Southern Africa College of Physicians. Henry has received support for his research from the Wellcome Trust, the Bill & Melinda Gates Foundation, the National Institutes of Health (USA) and the Medical Research Council (UK). He was Cornell University’s Distinguished African Scholar in 2015 and received the MRC/DfID African Research Leader Award in 2017. |
| 16:35 - 17:05 |
Streptococcus pneumoniae: life at the mucosal frontline
Despite the widespread use of pneumococcal conjugate vaccines (PCV), S. pneumoniae is still responsible for almost 300,000 deaths annually from pneumonia, meningitis and sepsis among children aged 1-59 months worldwide. In the face of the widespread roll-out of antiretroviral therapy, the burden of pneumococcal disease in HIV-infected adults has also remained considerable. Colonisation of the human nasopharynx is a pre-requisite for both pneumococcal disease and transmission, and has therefore been identified as a crucial target for vaccines. Remarkably, our understanding of the fundamental biology of pneumococcal colonisation in humans, and the determinants of the host response in healthy and vulnerable populations remains incomplete. The mucosal epithelium is a critical point of contact for a range of bacterial colonisers, transducing signals to recruit innate and adaptive immune pathways. HIV subverts pathogen-specific immunity at the mucosal frontline and disappointingly, the introduction of PCV into the routine schedule in Malawi has not achieved the herd protection of vulnerable populations seen in richer settings. We have found high rates of persistent invasive disease and residual nasopharyngeal carriage in HIV-infected adults. We have used a pneumococcal controlled human infection model to show that contrary to current thinking, Streptococcus pneumoniae invades the human mucosa during commensal carriage, triggering an epithelial innate-inflammatory response without causing disease. We have proposed that this so-called micro-invasion is fundamental to the outcome of colonisation and will describe how these findings may lead to novel interventions to interrupt S. pneumoniae carriage, and so disease and transmission. 
Dr Robert Heyderman, NIHR Global Health Research Unit on Mucosal Pathogens, University College London, UK

Dr Robert Heyderman, NIHR Global Health Research Unit on Mucosal Pathogens, University College London, UKRob Heyderman is a clinician scientist who bridges the gap between clinical practice, disease prevention and the fundamental understanding of infectious disease. He directed the highly successful Malawi-Liverpool-Wellcome Trust Programme (MLW) for over 8 years, transforming the Programme into a centre of research excellence and research training led by Malawian & international scientists, improving the health of people in sub-Saharan Africa. Since joining UCL, he has established a Mucosal Pathogens Research Group which pursues epidemiological, clinical and basic laboratory research in UK and Africa, addressing the microbial and immunological basis of severe infection caused by mucosal pathogens and their prevention through vaccination. He has recently launched the NIHR Global Health Research Unit on Mucosal Pathogens (MPRU), which is an interdisciplinary translational programme that brings together internationally recognised UK and African investigators to tackle limitations in the long-term effectiveness of existing vaccines to prevent meningitis, pneumonia and sepsis through new approaches to interrupting mucosal pathogen carriage/transmission. |
