Links to external sources may no longer work as intended. The content may not represent the latest thinking in this area or the Society’s current position on the topic.
Advances in antimicrobial innovation
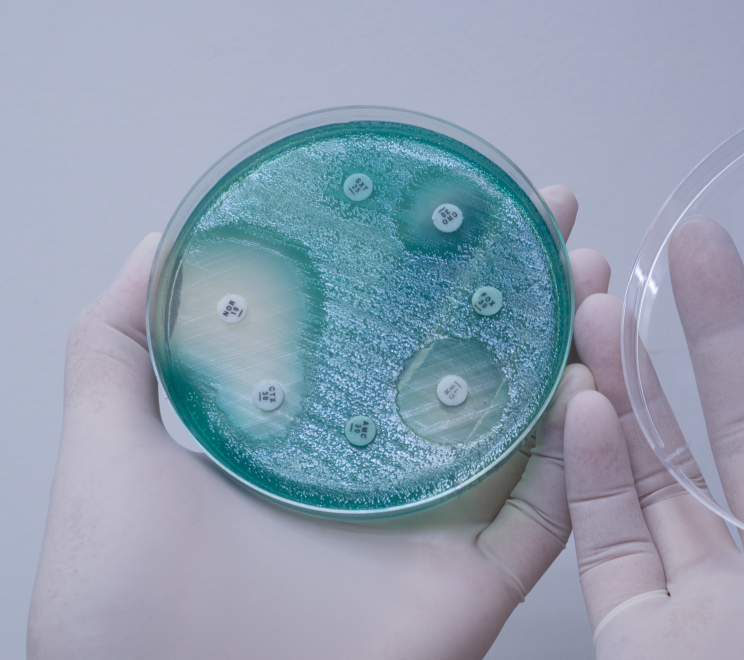
This joint Royal Society and Academy of Medical Sciences symposium brought together scientists, medical practitioners and funding bodies to raise awareness and interest for new anti-microbial advances.
A report on the event is available to read (PDF).
Background
Anti-microbial resistance continues to grow as a worldwide health challenge. Pathogens are continually evolving to combat drugs given to treat potentially life-threatening diseases, rendering treatments ineffective and threatening the many advances in fighting microbes over recent decades.
As therapeutics in current use become less effective, new approaches are required. This event aimed to raise awareness and interest for new anti-microbial advances, the challenges faced in market delivery, and their appropriate use in healthcare systems.
About the conference series
Supported by AstraZeneca, the meeting formed part of the Royal Society’s Transforming our future conferences in the life sciences, and the Academy of Medical Sciences’ FORUM programme. These meetings are unique, high-level events that address the scientific and technical challenges of the next decade. Each conference features cutting edge science from industry and academia and brings together leading experts from the scientific community, including regulatory, charity and funding bodies.
Organisers
Schedule
|
The future of AMR reimbursement: What types of products will succeed?
Companies successfully developing new antibiotics have collapsed after achieving approval due to the market failure that faces new antibiotics. This problem has been recognized and reimbursements that reflect the societal value of new antibiotics are coming. But, not all new antibiotics will earn a strong reward. Importantly, it is possible to Judge the likely value of a given project (e.g., the project you are working on right now!) and this talk will cover current insights on the future of AMR reimbursement. 
Dr John H. Rex, Chief Medical Officer, F2G Ltd.

Dr John H. Rex, Chief Medical Officer, F2G Ltd.Dr Rex is a physician and drug developer with more than 30 years of development and policy experience focused on antimicrobial agents. He is currently CMO for F2G, Ltd. (an antifungal biotech), is an operating partner with a venture capital group (Advent Life Sciences), and was (2015-2019) a voting member on the US Presidential Advisory Council on Combating Antibiotic Resistant Bacteria (PACCARB). He also blogs regularly at http://amr.solutions/blog.html. His experience includes moving compounds from early preclinical development through all development phases via academic positions (NIH, Bethesda, MD; Univ. of Texas Medical School-Houston) and VP-level roles at a multinational pharmaceutical firm (AstraZeneca). Other past activities include advancing novel regulatory paradigms for antibacterial agents, publications on novel reimbursement models for antibiotics, co-founding of a public-private partnership (CARB-X), co-founding of the New Drugs for Bad Bugs (ND4BB) program of Europe’s Innovative Medicines Initiative (IMI), and a 4-year term as Industry Representative on the FDA Anti-Infective Drugs Advisory Committee (AIDAC, 2007–2011). |
|
Antimicrobial Optimisation: Research Priorities, Advances in Innovation and Lessons from COVID-19

Professor Alison Holmes OBE FMedSci, Professor of Infectious Diseases, Imperial College London

Professor Alison Holmes OBE FMedSci, Professor of Infectious Diseases, Imperial College LondonAlison Holmes OBE FMedSci is Professor of Infectious Diseases and Director of both the NIHR Health Protection Research Unit in Healthcare Associated Infections and AMR and Centre for Antimicrobial Optimisation (CAMO), at Imperial College London. She leads a large international, multidisciplinary infectious disease research programme focusing on optimising antimicrobial use through the development of innovative approaches and technologies for the management and prevention of infections and the development of precision medicine, pioneering the integration of social sciences into research addressing antimicrobial resistance. Professor Holmes is an NIHR Senior Investigator and an Infectious Disease consultant within Imperial College Healthcare NHS Trust. She serves on WHO Expert groups related to antimicrobial use, AMR, infection prevention, sepsis, and COVID-19. She is the current President of the International Society for Infectious Diseases. |
|
Mining biosynthetic pathways for new antibiotics
One of the impediments to returning to microbial natural products as sources for leads for new antimicrobials is the rediscovery of known compounds when using traditional phenotypic extract screens. With the availability of thousands of microbial genome sequences, we now understand that such an approach does not comprehensively sample the potential of most microbes to produce new compounds. By directly exploring microbial genomes and identifying biosynthetic programs that have not yet been fully explored for their antibiotic potential, it is possible to select organisms and pathways that are likely to yield novel chemistry and antimicrobial activity. Coupled with synthetic biology tools to focus on these pathways, new antibiotics can be discovered. An example of our recent work using this genomes fist strategy coupled with a phylogenomic filter will be presented with a focus on new inhibitors of the ClpP protease. 
Professor Gerard Wright, DeGroote Institute for Infectious Disease Research, McMaster University

Professor Gerard Wright, DeGroote Institute for Infectious Disease Research, McMaster UniversityGerard (Gerry) Wright is the Director of the Michael G. DeGroote Institute for Infectious Disease Research and the David Braley Centre for Antibiotic Discovery and the inaugural academic lead of Canada’ Global Nexus for Pandemics and Biological Threats. He is a Professor in the Department of Biochemistry and Biomedical Sciences at McMaster University and holds the Michael G. DeGroote Chair in Infection and Anti-Infective Research and a Tier 1 Canada Research Chair in Antibiotic Biochemistry. He was elected as a Fellow of the Royal Society of Canada (2012) and a fellow of the American Academy of Microbiology (2013). His research interests are in the origins and mechanisms of antibiotic resistance and the discovery of new anti-infective strategies, in particular focusing on the application of microbial natural products and synthetic biology towards this goal. |
|
New methods of evaluating antimicrobials

Professor Colm Leonard, Consultant Clinical Advisor, NICE

Professor Colm Leonard, Consultant Clinical Advisor, NICE |
|
|
Innovation in Point of Care Diagnostics
AntiMicrobial resistance is a serious healthcare challenge, and it is estimated that drug resistant microbes will kill 10 million people worldwide per year by 2050. This has led researchers and governments to accelerate AMR research globally, primarily focusing on antimicrobial drug discovery to increase our arsenal of antimicrobials and preserve our working antimicrobials. Diagnostics have been highlighted in several reports as a key way of reducing overuse of antimicrobials in practice and encouraging rationalisation of antimicrobial prescribing. However, despite this, development of innovative diagnostic solutions has been overlooked and underfunded, likely due to the "blue sky" nature of this type of research. Dr Joshi’s research focusses on innovative, interdisciplinary solutions for diagnosis of AMR infections at point of care. This talk will focus on AMR, links to Climate change and the importance of encouraging and investing in new, feasible, long term approaches to tackle AMR. 
Dr Tina Joshi, Lecturer in Molecular Microbiology, Faculty of Health, University of Plymouth

Dr Tina Joshi, Lecturer in Molecular Microbiology, Faculty of Health, University of PlymouthDr Tina L. Joshi is a Lecturer in Molecular Microbiology at the University of Plymouth. She has been nominated for and won several national and international awards related to her teaching and research. Her specialisms are Clinical Microbiology and Infectious Diseases, with a strong focus on interdisciplinary research to help tackle the global Healthcare Challenge of Anti-Microbial Resistance. Her research spans Infection Prevention and Control, and development of innovative Molecular Diagnostic solutions for pathogens at point of care, utilising engineering, physics and microbiology. She has extensive experience of translational research collaborations with clinical and industry partners and has patented her research. She regularly contributes to national policy reports and AMR outreach in local and national media, and is a member of the Science Committee of Antibiotic Research UK, the Microbiology Society Impact and Influence committee and is Chair-Elect of the Federation of Infection Societies. |
|
|
How can novel clinical trial designs address challenges in evaluating antimicrobials for multi-drug resistant organisms?
The paradigm of evidence-based medicine has been to optimise outcomes by iteratively improving a "standard-of-care" (SOC) regimen, which forms the basis for comparison with new interventions. Platform trials generally still follow this paradigm, comparing multiple new interventions to SOC, but speed up drug development by doing this simultaneously. However, this paradigm poses multiple challenges for antimicrobials, in particular the absence of a clear SOC, with large numbers of antimicrobials with a week evidence base supporting their use. A single SOC will drive resistance, supporting diversity in prescribing – but comparisons vs different "SOC" require multiple large non-inferiority trials which are simply infeasible. I will review two recent developments in trial design which could be exploited to address these challenges, and highlight the considerable challenges that still remain. 
Professor Ann Sarah Walker FMedSci, Professor of Medical Statistics & Epidemiology, University College London

Professor Ann Sarah Walker FMedSci, Professor of Medical Statistics & Epidemiology, University College LondonSarah Walker (FMedSci, NIHR Senior Investigator) is Professor of Medical Statistics and Epidemiology at the Medical Research Council Clinical Trials Unit at University College London (MRC CTU at UCL) (40%) and at the Nuffield Department of Medicine at Oxford University (60% FTE). At UCL she has responsibility for the statistical design, management and analysis of a portfolio of randomised controlled trials and other interventional and non-interventional studies in the field of infectious diseases, particularly HIV, Hepatitis C, the acutely sick child in Africa and bacterial infections, including as Trial Statistician for 15 randomised trials in high-, middle- and low-income countries over the last 10 years. She has a track record in applying efficient but complex and challenging designs, including factorial and multi-arm multi-stage, to address multiple questions within each trial. |
|
The phage approach

Dr Heather Fairhead, CEO, Phico Therapeutics

Dr Heather Fairhead, CEO, Phico Therapeutics |
Chair

Dr Flic Gabbay FMedSci, Managing Partner, tranScrip

Dr Flic Gabbay FMedSci, Managing Partner, tranScrip
Flic is the Managing Partner and co-founder of tranScrip. A pharmaceutical physician, she has more than 30 years of industry experience and has held senior positions in big pharma, biotech and CROs in both Europe and North America.
Flic is an expert in Early Drug Development. She has extensive experience of working on the development, submission and launches of products in infection, respiratory disease, arthritis, immunology, chronic kidney disease and oncology.
Flic is a committed entrepreneur and was founding Chairman of the steering group that set-up the UK Faculty of Pharmaceutical Medicine (FPM) and is currently Vice President and President Elect. In 2020, Flic was awarded the Fellowship of the Academy of Medical Sciences.

Dr Erin Duffy, Chief of Research & Development, CARB-X, Boston University School of Law

Dr Erin Duffy, Chief of Research & Development, CARB-X, Boston University School of LawErin Duffy is the Chief of Research & Development at CARB-X. CARB-X is a global non-profit partnership dedicated to accelerating antibacterial research to tackle the global rising threat of drug-resistant bacteria. With up to US$480 million to invest in 2016-22, CARB-X funds the world’s largest early development pipeline of new antibiotics, vaccines, rapid diagnostics, and other products to prevent, diagnose and treat life-threatening bacterial infections. Prior to CARB-X, she worked at Melinta Therapeutics (fka Rib-X Pharmaceuticals) for 17+ years, where she became EVP, Chief Scientific Officer, leading the research and early development activities of the Company. She began her industrial career at Pfizer Central Research. Erin holds a PhD from Yale University in physical-organic chemistry. 
Dr Adam Zerda, Director AMR Strategy and Development, Becton, Dickinson and Company (BD)

Dr Adam Zerda, Director AMR Strategy and Development, Becton, Dickinson and Company (BD)Adam Zerda is Director, Antimicrobial Resistance (AMR) Strategy and Development for BD. In this role, Adam is responsible for both internal and external efforts to build awareness of AMR, engage stakeholders in policy and advocacy efforts around AMR, and deploying training and process improvement programs with customers, focusing specifically on infection prevention and antimicrobial stewardship. BD’s AMR program is cross-functional and company-wide, requiring close alignment between businesses and functions in all regions where BD operates. Adam has a Ph.D. in Polymer Science and Engineering and a B.S. in Chemistry. He is based in North Carolina where he additionally sits on the board of North Carolina BIO. Adam has previously held role in R&D, strategy and innovation at BD. 
Dr Michael Gutch, Chief Financial Officer and Chief Business Officer, Entasis Therapeutics

Dr Michael Gutch, Chief Financial Officer and Chief Business Officer, Entasis TherapeuticsMichael Gutch, PhD, has served as chief business officer and chief financial officer of Entasis Therapeutics since April 2017. From January 2014 to March 2017, he served as executive director of corporate development and head of equities at AstraZeneca. Dr Gutch served as managing director, MedImmune Ventures, the corporate venture capital arm of AstraZeneca, from September 2011 to December 2013. Prior to that, Dr Gutch served as investment director of HIG BioVentures at the investment firm HIG Capital and as a principal of Lilly Ventures, the corporate venture arm of Eli Lilly & Company. He currently serves on the boards of directors of Albireo Pharma, Inc. Dr Gutch received his MBA in Finance from Indiana University and a PhD in Molecular Pathology from SUNY Stony Brook. He earned his BS degrees in Biology and Chemistry from Alfred University. 
Professor Angharad Davies, Swansea University Medical School/Vice-President for Learning, Royal College of Pathologists

Professor Angharad Davies, Swansea University Medical School/Vice-President for Learning, Royal College of PathologistsAngharad Davies is professor and consultant medical microbiologist at Swansea University Medical School, and Vice-President (Learning) for RCPath. She has wide experience in education around antimicrobial resistance and stewardship. As well as leading on medical student infection and immunity teaching at Swansea she runs a module for bioscience students on antimicrobial resistance, a number of postgraduate modules, and an interprofessional course on antimicrobial resistance and stewardship for specialist healthcare practitioners such as antimicrobial pharmacists. She is a Principal Fellow of the Higher Education Academy. Angharad founded and leads the All-Wales Antimicrobial Resistance Educators (AWARE Wales) group, a virtual interdisciplinary network supporting education on resistance and stewardship in NHS Wales by sharing best practice and resources. She is a co-author of the NICE-endorsed antimicrobial resistance and stewardship competencies for UK undergraduate medical students and is Health and Care Research Wales’ specialty lead for infection. Angharad sits on the project board for the Pathology Portal, a major digital educational collaboration between RCPath and Health Education England. |
|
Innovating to secure the future of modern medicine
Antibiotics are essential infrastructure for our health systems. But declining R&D into new antibiotics compounded by increasing shortages of existing generics, has left fewer drugs left in our medicine cabinet. Meanwhile, our existing drugs are no match for superbugs. This lack of innovation compromises access for patients, and undermines modern medicine for every country. Working together, in the context of drug development, and to change the politics around it, must happen across countries and across sectors. To turn the tide on the slow pandemic of AMR, we need to bring research, development, policy and politics together, with stewardship, access and innovation at the heart. Dame Sally Davies will reflect on key global advances in antibiotic innovation, and what the world still needs to do to win the war against superbugs. 
Professor Dame Sally Davies, UK Special Envoy on Antimicrobial Resistance, UK Government

Professor Dame Sally Davies, UK Special Envoy on Antimicrobial Resistance, UK GovernmentDame Sally Davies was appointed as the UK Government’s Special Envoy on AMR in 2019. She is also the 40th Master of Trinity College, Cambridge University. Dame Sally was the Chief Medical Officer for England and Senior Medical Advisor to the UK Government from 2011-2019. She is a leading figure in global health, having served as a member of the World Health Organisation (WHO) Executive Board 2014-2016, and as co-convener of the United Nations Inter-Agency Co-ordination Group (IACG) on Antimicrobial Resistance (AMR), reporting in 2019. In November 2020, Dame Sally was announced as a member of the new UN Global Leaders Group on AMR, serving alongside Heads of State, Ministers and prominent figures from around the world to advocate for action on AMR. In the 2020 New Year Honours, Dame Sally became the second woman (and the first outside the Royal family) to be appointed Dame Grand Cross of the Order of the Bath (GCB) for services to public health and research, having received her DBE in 2009. |


