Links to external sources may no longer work as intended. The content may not represent the latest thinking in this area or the Society’s current position on the topic.
Biological and climatic impacts of ocean trace element chemistry
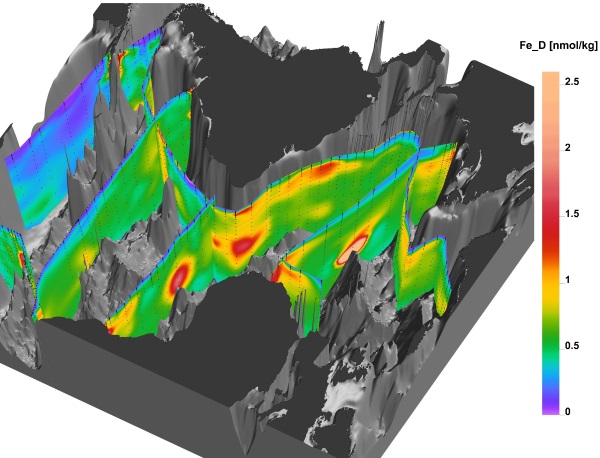
Scientific discussion meeting organised by Professor Gideon Henderson FRS, Professor Ed Boyle, Professor Maeve Lohan, Dr Micha Rijkenberg and Dr Géraldine Sarthou.
Trace metals are critical for life, are toxic pollutants, and are tracers of process and rate in the past and present ocean. Significant recent measurement campaigns have revolutionised understanding of the oceanic cycling of trace elements. This meeting will discuss these advances, and their implications for a broad range of ocean, climate, and carbon cycle research.
You can download the draft programme (PDF), and biographies of the organisers and speakers are available below, together with speaker abstracts.
Attending this event
This meeting has already taken place. Recorded audio of the presentations can be found below, and papers from the meeting will be published in a future issue of Philosophical Transactions A.
This meeting was immediately followed by a related, two-day satellite meeting Quantifying fluxes and processes in trace-metal cycling at ocean boundaries, at the Royal Society at Chicheley Hall, home of the Kavli Royal Society Centre.
Enquiries: Contact the events team
Organisers
Schedule
| 09:05 - 09:30 |
Trace elements in seawater: advances from global measurement campaigns
Global campaigns began mapping the physical structure of ocean water masses nearly a century ago. Each major water mass can be linked to its region of formation through unique ranges of temperature and salinity. A half-century later the global distributions of chemical tracers of ocean circulation, measured together with the chemical byproducts of photosynthesis and respiration by the GEOSECS programme, illustrated the well-choreographed interplay between physics and biology in regulating the distributions of carbon and nutrients in the ocean. A revolution in marine analytical chemistry, beginning in the 1970’s, provided the first glimpse of oceanographically consistent distributions of many trace elements and their isotopes. However, despite the heroic efforts of these chemists, a global picture of the distributions of trace elements remained out of reach for more than a quarter century. Building on the GEOSECS legacy, the GEOTRACES programme was designed to remedy this situation by coordinating the efforts of marine geochemists worldwide to identify processes and quantify fluxes that control the distributions of key trace elements and isotopes in the ocean. Fulfilling this mission will benefit other areas of ocean research, and the public at large, by defining the sources of trace nutrients that regulate ocean fertility and ecosystem health, the fate of many contaminants in the ocean, and the role of the ocean in past climate change. This presentation will begin with selected examples of new insights gained from basin-wide distributions of trace elements and their isotopes. The second part will illustrate how the wealth of trace element and isotope data generated by GEOTRACES allows one to constrain the supply of trace elements using multiple independent techniques. This approach to the synthesis of GEOTRACES data will use aerosol (dust) deposition for illustration and segue into the next presentation. 
Professor Robert Anderson, Lamont-Doherty Earth Observatory of Columbia University, USA

Professor Robert Anderson, Lamont-Doherty Earth Observatory of Columbia University, USARobert Anderson received his PhD in chemical oceanography from the Massachusetts Institute of Technology – Woods Hole Oceanographic Institution joint program in oceanography. He is a Ewing-Lamont Research Professor at Lamont-Doherty Earth Observatory, and an Adjunct Professor in the Department of Earth and Environmental Sciences, Columbia University, USA. His research interests include the marine biogeochemical cycles of trace elements and their isotopes as well as the ocean carbon cycle, and its sensitivity to climate variability. He was a founding co-chair of the International GEOTRACES Program (2007-2011). Anderson is a Fellow of the American Geophysical Union and of the Geochemical Society. See additional information at: http://www.ldeo.columbia.edu/user/boba. |
|
|---|---|---|
| 09:45 - 10:15 |
Atmospheric transport to the oceans of trace elements and micronutrients
The atmosphere is now known to be an important route for the supply of various trace elements from the land to the ocean. The trace elements transported can include key micro and macro nutrients, as well as some pollutants and tracers. The transport of iron within crustal dust has particularly important biogeochemical impacts, but other trace elements may also play an important biogeochemical role. The solubility of trace elements in aerosols varies greatly. This partly reflects emission processes, with trace elements associated with high temperature (generally anthropogenic) emission processes tending to be more soluble than those associated with low temperature processes (such as dust transport from deserts). In addition solubility of some trace elements bound within aluminosilicate lattices appears to vary systematically across the globe, with highest solubilities in areas of lowest dust loadings, although the controls on these gradients are not well understood. In this presentation we will discuss the general patterns of atmospheric trace element transport, focussing particularly on the Atlantic Ocean, but also considering other ocean areas. We will also consider the patterns of trace element solubility in atmospheric aerosols and the possible solubility control mechanisms, deposition fluxes and the uncertainties associated with these, and finally touch on the biogeochemical impacts in so far as we know them now. 
Professor Tim Jickells, University of East Anglia, UK

Professor Tim Jickells, University of East Anglia, UKTim Jickells is an environmental chemist with research interest in coastal nutrient cycling and atmospheric inputs to the oceans and their impact. His research has involved extensive field campaigns particularly in the Atlantic from Iceland to Antarctica, and also in the Pacific. After a BSc in Chemistry and an MSc in Oceanography, Tim began his working life in Glasgow with the Clyde River Purification Board before moving to Bermuda where he completed his PhD research, and then moved to UEA in 1985 where he is now a Professor. He was the NERC Theme Leader for Earth System Science 2007-2012, was awarded the Challenger medal by the Challenger Society in 2006 and became a Fellow of the Royal Society of Chemistry in 2012. |
|
| 11:00 - 11:30 |
Solid river inputs and ocean margins as critical sources of elements to the oceans
Land to ocean transfer of material largely controls the chemical composition of seawater and the global element cycles. Compilation of results on radiogenic isotopic tracers (Sr, Th, Nd) suggest that a significant fraction of the particulate material discharged by the rivers and deposited on the shelves and slopes, dissolves in seawater after its arrival in the oceans. Moreover, data on non-traditional isotopes acquired in the framework of GEOTRACES (for example Ni, Mo or Zn) reveal that the oceanic budget of these elements is not balanced. The mass of most elements arriving in the ocean via particulates exceeds that arriving via dissolved transport by at least a factor of 50. Consequently, we hypothesise that particulate material dissolution in the oceans may be the dominant mechanism contributing numerous elements to the ocean. We also suggest that estimates based on dissolved riverine transport alone may significantly underestimate the global element fluxes in the oceans. Discussing this issue is particularly interesting in the Atlantic Ocean, which is also subjected to the largest fresh water and dust inputs of the world (Amazon and Saharan respectively). 
Professor Catherine Jeandel, CNRS, Observatoire Midi-Pyrenees, France

Professor Catherine Jeandel, CNRS, Observatoire Midi-Pyrenees, FranceOceanographer and marine geochemist, Catherine Jeandel’s research objectives are focused on quantifying the fluxes and processes that govern the chemical state of the ocean. She stands among the pioneers in developing trace elements and their isotope analyses for seawater and marine particles, which she measured as part of many different cruises in the world ocean. Because one single tracer is not sufficient enough to constrain complex natural systems, she joined other marine geochemists to advocate multi-tracer approaches to resolving natural processes, yielding to the building and launching of the international GEOTRACES program (2010). After a master and PhD thesis in marine geochemistry at University of Paris VII, Catherine Jeandel obtained a research position at CNRS, were she is presently Research Director. She moved in 1985 to the University of Toulouse, where she is still working in the “Toulouse Isotopie Marine” research group of the Laboratoire d’Etudes en Geophysique et Océanographie Spatiale (LEGOS). |
|
| 11:45 - 12:15 |
Modelling the transport of trace elements: the mantle He example
One of the most exciting discoveries of the GEOTRACES observational programme are the pronounced deep-ocean trace element plumes originating from hydrothermal vents along geologically active ocean ridges found in all ocean basins. The shape and extent of the plumes, as well as the actual concentrations found in the plumes are the combined result of biogeochemical and physical processes, with water-mass circulation and mixing playing a crucial role. Inferences about source strengths or reaction rates in the plumes therefore depend on knowledge of the physical transports and often require the use of models. In this talk I concentrate on the ‘classic’ example of mantle He that is released together with the trace elements along deep ocean ridges, but, as a noble gas, is not affected by complicating chemical reactions. A global ocean model with realistic chlorofluorocarbon and radiocarbon distributions is used to simulate distributions of 3He and 4He. In agreement with observations this model produces separate westward spreading He plumes south and north of the Equator in the deep eastern Pacific. Results for adapted He source strengths and pathways of the mantle He to the ocean surface are presented. These results help the interpretation of the more complicated biogeochemically active trace elements, such as Fe, Zn, Mn and Al. 
Professor Reiner Schlitzer, Alfred Wegener Institute, Germany

Professor Reiner Schlitzer, Alfred Wegener Institute, GermanyReiner Schlitzer obtained a Diploma in Physics and a PhD in Environmental Physics at the University of Heidelberg. He held a post-doctoral position at the Massachusetts Institute of Technology, habilitated at the University of Bremen and then moved to the Alfred Wegener Institute in Bremerhaven. Schlitzer has been Professor for Environmental Physics at the University of Bremen since 2003. He studies the biogeochemical cycles of oxygen, nutrients, carbon and other tracers in the ocean and has worked as observationalist, producing the first radiocarbon section in the East Atlantic and as modeller, developing and using inverse models for the quantification of marine production, carbon uptake and transport, as well as fluxes and remineralisation of particles. He is the developer of the Ocean Data View software and the creator of the eWOCE and eGEOTRACES electronic atlases. |
| 13:30 - 14:00 |
Local and global-scale interactions between micro-nutrient cycles and phytoplankton
At the local scale, Resource Ratio Theory suggests that the relative supply rates of key resources control community composition and nutrient concentration. Interpreting global-scale data sets with this framework we find clear relationships between the relative rates of delivery of iron, nitrogen and phosphorus to the surface ocean and the biogeography of nitrogen fixation. Considering the global-scale, we hypothesise a feedback that maintains marine trace metal abundances to be co-limiting with macro-nutrients over long times-scales, suggesting that there is ‘just enough’ iron in the ocean. 
Dr Mick Follows, Massachusetts Institute of Technology, USA

Dr Mick Follows, Massachusetts Institute of Technology, USAMick Follows is an oceanographer in the Department of Earth, Atmospheric and Planetary Sciences at MIT. He seeks to understand how the interactions of physical, chemical and biological processes modulate the structure and function of marine microbial communities and regulate elemental cycles on the global scale. He enjoys using simple models to describe key processes and interactions from the cellular to the global scale. He employs numerical simulations to illustrate how these processes play out in complex environments. |
|
|---|---|---|
| 14:15 - 14:45 |
Ecosystem responses to dust and ash input to the surface ocean
Atmospheric transport and deposition of land derived particulate material represents a key connection pathway between terrestrial and oceanic systems. In particular, atmospheric deposition of both desert dust and volcanic ash can influence oceanic systems through providing upper ocean ecosystems with a range of nutrients, including biologically essential trace metals such as iron. Emphasising the multi-nutrient characteristics of particle associated inputs, alongside microbial nutritional requirements, this talk will briefly review evidence for significant impacts of dust and ash on marine ecosystems using a combination of results from small scale experiments, observations of discrete events and analysis of apparent relationships between biogeochemical tracers in the context of simple theory and numerical models. A distinction is drawn between the short term regional impacts which may result from discrete episodic event scale processes and apparent large scale biogeochemical partitioning of the upper ocean which is argued to partially reflect geographical variations in major aerosol sources. However, we argue that the consequences of predominantly event scale ash deposition and more chronic dust borne nutrient inputs both need to be considered in the context of large scale spatial variability in oceanic nutrient deficiency, as can be illustrated through analysis of the new data becoming available through the GEOTRACES programme. 
Dr Mark Moore, University of Southampton, UK

Dr Mark Moore, University of Southampton, UKDr Mark Moore is a Reader in Ocean and Earth Science at the University of Southampton based at the National Oceanography Centre Southampton. His research interests focus on understanding how linkages between the integrative metabolic activities of complex marine microbial activities, which emerge in response to multiple environmental drivers, interact with the global nutrient and carbon cycles. Dr Moore undertakes his research using a combination of at sea observations, in situ and laboratory experimentation and simple numerical modelling. He works on the cycling of both macronutrients and trace metals, being particularly interested in linkages between these cycles through microbial processes. He is particularly interested in the impact of atmospheric dust sources on diazotrophy and consequential impacts on ocean biogeochemistry. |
|
| 15:30 - 16:00 |
Hydrothermal inputs of micronutrients as a control on ocean productivity
Hydrothermal vents were only discovered in the late 1970s thanks to advances in undersea technology. Around the same time, new sampling techniques demonstrated that the levels of the micronutrient iron in the ocean were much lower than previously thought. From this the now well established role of iron in shaping the distribution and magnitude of ocean productivity emerged. After their discovery, hydrothermal systems were quickly acknowledged as having extremely high iron concentrations but, equally, their influence on the oceanic iron inventory was quickly dismissed as negligible. In recent years a combination of observational and modelling studies have led to this paradigm being reversed and hydrothermal activity is being recognised as an important component of the ocean iron cycle. As iron regulates biological activity over large parts of the ocean, hydrothermal inputs may therefore play an important role in shaping the distributions and magnitude of ocean productivity in regions such as the Southern Ocean. Recent results highlight the relative importance of different ocean ridge systems in sustaining rates of ocean productivity that can direct future fieldwork. In this talk I will review recent developments in this rapidly expanding field and highlight the key emerging questions. 
Professor Alessandro Tagliabue, University of Liverpool, UK

Professor Alessandro Tagliabue, University of Liverpool, UKProfessor Tagliabue is a Professor at the University of Liverpool and an ocean biogeochemist, interested in how the cycling of resources in the sea affects biological activity and vice-versa. He is particularly interested in trace micronutrients and how they interact together to shape primary production, ecosystem structure and their response to global change. His science links numerical models, at both global and idealised scales, with both fieldwork and synthesis of datasets. Professor Tagliabue is heavily involved in the international GEOTRACES programme, was a lead author on the IPCC Special Report on Oceans and Cryosphere in a Changing Climate and is a member of the governing council of the UK Challenger Society for Marine Science. He is also UK Chair for SCOR and sit on the Royal Society Global Environment Research Committee.
|
|
| 16:15 - 16:45 |
Ecosystem controls on the oceanic cycling of trace metals
Marine phytoplankton (microscopic algae) annually convert 45 gigatons of CO2 to organic carbon via photosynthesis in surface waters. This primary production sustains the majority of life in the oceans, and plays a critical role in the global carbon cycle. Over the past two decades, there has been significant research interest in trace metals (e.g. Fe, Cu and Co) as important controls on marine productivity, and a growing awareness that plankton can greatly influence oceanic trace metal cycling. In this talk, I will illustrate how the marine biogeochemical cycling of trace metals is closely linked to ecosystem functioning. I will first review key mechanisms by which plankton affect the concentration, distribution and speciation of trace elements in the sea. For example, phytoplankton can concentrate bioactive metals intracellularly by more than a million fold relative to dissolved concentrations in seawater, and can influence the redox speciation of metals through cell-bound oxidases and reductases. I will also present recent findings describing how organisms in upper trophic levels (e.g. krill, whales and seabirds) concentrate, retain and excrete trace elements, thus impacting the cycling and residence time of metals in surface waters. Emerging analytical techniques are providing information on the differential trace metal stoichiometry of organisms across entire food webs, while traditional methods have provided valuable data on metal assimilation efficiencies in many organisms. By incorporating these data in mass balance ecosystem models, we are now well poised to significantly advance our understanding of ecosystem-level controls on trace metal cycling in the ocean. 
Dr Maite Maldonado, University of British Columbia, Canada

Dr Maite Maldonado, University of British Columbia, CanadaMaite Maldonado is a biological oceanographer investigating the physiological adaptations of bacteria and microscopic algae inhabiting oceanic regions with extremely low trace metal concentrations. Her research interests include how microbes affect the concentrations and speciation of trace metals in marine surface waters, and how, in turn, the bioavailability of trace metals controls primary productivity in the ocean. Her research involves field campaigns in the NE Subarctic Pacific Ocean, the coastal upwelling in Central California, the Arctic Ocean, as well as the Southern Ocean. Maite completed a BA in Biology and Marine Sciences at Smith College (Massachusetts, USA), followed by a PhD at McGill University (Montreal, Canada). After a post-doctoral project investigating nitrogen and trace metal cycling in the permanently ice covered, saline Lake Bonney in the Dry Valleys of Antarctica, Maite moved to the University of British Columbia (Vancouver) in 2002, where she is a Canada Research Chair (TierII)/Associate Professor. |
| 09:00 - 09:30 |
Radiogenic isotope tracers of present and past ocean circulation
The deep ocean contains ~60 times more carbon than the atmosphere and a large part of the carbon exchange takes place in areas of deep water formation and upwelling. Therefore small changes in the rate of deep water formation are likely to have a large impact on the atmospheric carbon budget. Hence it is – and has been – of great interest to decipher the relationship between climate change and ocean circulation. While we have ample ways to look at ocean circulation in the modern ocean, we struggle to reconstruct physical oceanography in the past. Neodymium (Nd) isotopes have been considered a promising ‘quasi-conservative’ water mass tracer for many years, and have been used extensively to reconstruct ocean circulation, on million year, glacial-interglacial, and even more rapid (sub)millennial timescales. While published Nd isotope records show intriguing co-variations with other climate indicators, our understanding of the modern cycle of Nd in the ocean has been rather poor. A good understanding of modern processes is, however, critical before a palaeo tracer can be applied with confidence to study the past. In this talk I will assess where we stand on using Nd isotopes as a palaeo-circulation tracer by scrutinising the wealth of new data collected on modern seawater in the context of the international GEOTRACES programme, which more than doubled the observational database over the past five years. Evaluating this data set reveals critical and novel information to be considered for palaeo interpretations, with implications on the ocean’s role in climate change. 
Dr Tina van der Flierdt, Imperial College London, UK

Dr Tina van der Flierdt, Imperial College London, UKTina van de Flierdt is Reader in Isotope Geochemistry at the Department of Earth Science and Engineering at Imperial College London. She is a geologist by training, whose academic background includes a PhD in Natural Science from the ETH Zurich, Switzerland, and a postdoctoral Fellowship and Associate Research Scientist position at Lamont-Doherty Earth Observatory of Columbia University, USA. She co-leads the MAGIC isotope facility at Imperial College London. Her research spans a variety of fields from understanding chemical cycles of trace elements in the ocean, over past ocean circulation and climate relationships, to the history of continental ice sheets and their vulnerability to future climate change. She utilises radiogenic isotopes to unravel these topics, and is a member of the UK GEOTRACES steering committee and the international GEOTRACES Standards and Intercalibration committee. |
|
|---|---|---|
| 09:45 - 10:15 |
Transition metal isotopes as tracers of oceanic metal budgets and cycling
The GEOTRACES programme has come at a propitious time, when we are newly able to study trace metal isotope variations in seawater. This contribution will review the progress of this new endeavour, with an emphasis on two distinct but related applications: (1) One of the most dramatic features of ocean chemistry is the extreme transfer of metals from the surface to the deep associated with nutrient-like behaviour. For some metals this internal cycling is associated with isotope fractionation. But for others even near complete uptake in the photic zone does not come with a significant isotope effect. An understanding of this dichotomy can only emerge from a more active pursuit of process studies that focus on phytoplankton uptake mechanisms and their controls, including the interplay between different trace metals. We suggest that some oceanic hubs, in particular the high-latitude oceans, are proving to be just as key to trace metals as they are for major nutrient distributions. (2) Isotope systems can help us to quantify and understand whole ocean transition metal budgets, including inputs and outputs, and the processes that control them. In a larger-scale perspective an important emerging theme concerns the contrasting isotopic impact of open ocean, oxic, versus marginal, anoxic/sulphidic, sinks from the dissolved pool to sediment. The contrast has implications for understanding ocean chemistry in deep time, and may turn out to be one of the more important applications of newly-developing transition metal isotope systems in earth science. 
Professor Derek Vance, ETH Zürich, Switzerland

Professor Derek Vance, ETH Zürich, SwitzerlandDerek Vance is Professor of Geochemistry at the Department of Earth Sciences, ETH Zürich, Switzerland. His research interests encompass many aspects of the geochemistry of the surface Earth, throughout Earth history from the Archaean onwards. His principal overall research direction today concerns the linkages between the various large-scale chemical cycles at the surface of the Earth. Specific research topics include the relationships between the long-term carbon cycle and chemical weathering, links between the short-term carbon cycle and oceanic trace metal chemistry, and the history of oxygen in the atmosphere. He has always been particularly keen on developing new geochemical and isotopic tools, such as metal isotope systems in the ocean, to learn more about the past environment on Earth. |
|
| 11:00 - 11:30 |
U-series rate-meters for ocean processes
The distribution of trace-elements in the ocean is controlled by their input, removal and internal biogeochemical cycling. Assessing the rates at which these processes move trace metals is fundamental to understanding the modern distribution, the supply of trace metals to photic surface waters, and the response of ocean biogeochemistry to change. The differential solubility of the isotopes in the U and Th decay chains, coupled to their wide range of half-lives, provide many tools with potential to quantify the rates of processes involved in trace-metal cycling. Insoluble Th has recently been shown to be a tracer for dust input, combining long-lived 232Th with shorter-lived 230Th to quantify the rates of modern dust addition averaged over a few years. The 230Th removed from seawater accumulates in underlying sediment where it provides a proxy for the rate of sedimentation and of sediment dissolution, and is used to normalise sedimentary 231Pa concentration to provide a controversial proxy for the rates of past ocean circulation. The shorter lived 234Th can be used to assess the downward flux of trace elements due to biological producitivity. Soluble isotopes of Ra also provide powerful rate information, particularly related to the rates of mixing and advection in the modern ocean. This talk will overview recent and new data illustrating the power of U-series isotopes to quantify the operation of ocean trace-element cycles. 
Professor Gideon Henderson FRS

Professor Gideon Henderson FRSGideon Henderson FRS became Defra’s Chief Scientific Adviser from October 2019 and is a Professor of Earth Sciences at the Department of Earth Sciences, University of Oxford. He is also a Senior Research Fellow at University College, Oxford and an Adjunct Associate Research Scientist at the Lamont Doherty Earth Observatory of Columbia University. In 2013, he was elected a Fellow of the Royal Society. His research uses geochemistry to understand surface earth processes, particularly those relating to climate, the ocean, and the carbon cycle. |
|
| 11:45 - 12:15 |
Nitrogen isotope variations as tracers of marine nitrogen biogeochemistry
Nitrogen (N) isotopes are a powerful tool for tracking N cycling in past and present marine environments. Many of the biogeochemical processes that comprise the marine N cycle lead to isotopic fractionation between pools of N, and the intensities of these activities are recorded in the isotope ratios of the substrates and products of the reactions. Denitrification, the microbial reduction of nitrate to nitrite and gaseous products, occurs in subsurface oxygen deficit zones (ODZs) and is a dominant process in the marine N budget. Its activity leads to removal of fixed (bioavailable) N, potentially lowering marine productivity on a variety of space and time scales. Denitrification also leads to enrichment of 15N in nitrate, and the effects of this process can be observed long distances from ODZs. These signals can be mixed and advected out of the ODZ, or may be upwelled to the surface and incorporated into particulate organic matter. This transfer of ODZ signals into phytoplankton biomass and preservation in sediments leads to historical records of the intensity of water column denitrification where N uptake in surface waters is complete. N isotope data in nitrate and nitrite from a series of cruises to the Eastern Tropical South Pacific (ETSP), including GEOTRACES GP16, are used to more closely investigate the pathways of N transformation in the ETSP, as well as the transmission and alteration of N isotope signals beyond the oxygen deficient waters. 
Dr Karen Casciotti, Stanford University, USA

Dr Karen Casciotti, Stanford University, USAKaren Casciotti is an Associate Professor in the Earth System Science Department at Stanford University. She received a Bachelor’s degree in Environmental Engineering Science at the California Institute of Technology, a Master’s degree in Oceanography from the Scripps Institution of Oceanography, and a PhD in Geosciences from Princeton University. She has participated in research cruises in the Pacific, Indian, and Southern Oceans. She has served as the nitrogen isotope intercalibration coordinator for GEOTRACES and currently serves on the US GEOTRACES Steering Committee and the Standards and Intercalibration Committee for GEOTRACES. Her current research focuses on marine nitrogen cycle biogeochemistry, with an emphasis on using nitrogen and oxygen isotopes to understand how nitrogen is cycled in oceanic suboxic zones. In addition, she teaches Marine Chemistry and Marine Stable Isotopes at Stanford. |
| 13:30 - 14:00 |
Trace metal biogeochemistry and bioavailability in an acidifying ocean
Because it directly affects chemical speciation, the acidification of the ocean influences the bioavailability of trace elements and their biogeochemical cycling. Here we focus on two biologically essential trace metals, iron (Fe) and zinc (Zn). The limited laboratory and field data available indicate that the availability to phytoplankton of Fe decreases with decreasing pH while that of Zn sometimes decreases and sometimes increases. The decrease in Fe bioavailability at low pH is often fully explained by the change in Fe speciation, namely the decrease in the concentration of the Fe(OH)n(3-n)+ species. But when all the Fe is bound to strong chelating agents (and remains so as the pH decreases), it is presumably the response of the Fe uptake system of phytoplankton to acidification that is responsible for the change in bioavailability. For Zn, which does not form stable hydroxide complexes at circumneutral pH, the decrease in binding to organic chelators at low pH should increase Zn bioavailability as is seen in some field and many laboratory experiments. However, the formation of bioavailable weak Zn complexes can reverse this trend. Both the increase and decrease in Zn bioavailability at decreasing pH reflect a change in chemical lability that can be quantified by electrochemistry. 
Professor François Morel, University of Princeton, USA

Professor François Morel, University of Princeton, USAFrançois M M Morel is the Albert G Blanke Professor of Geosciences at Princeton University. He received a B.S. in Engineering from the University of Grenoble, France, and a PhD in Engineering Science from the California Institute of Technology. He was a faculty member at the Massachusetts Institute of Technology from 1973 to 1994 and joined the Princeton faculty in 1994. The research in his laboratory focuses on the interaction of trace metals and microorganisms in the environment, with particular emphasis on the role of metals in the global cycles of carbon and nitrogen in marine and terrestrial systems. Morel is a member of the National Academy of Sciences and has received awards from the American Geophysical Union, the Geochemical Society, the American Chemical Society, the California Institute of Technology, Carnegie Mellon University, and the Eni Foundation. |
|
|---|---|---|
| 14:10 - 14:40 |
The ocean mercury cycle and its anthropogenic perturbation
The toxic metal mercury is present only at trace levels in the ocean, but it accumulates in fish at concentrations high enough to pose a threat to human and environmental health. Human activity has dramatically altered the global mercury cycle, resulting in loadings to the ocean that have increased by at least a factor of three from pre-anthropogenic levels, and these loadings may continue to increase as a result of higher atmospheric emissions and other factors related to global environmental change. The impact that these new loadings, as well as previously released ‘legacy’ mercury, will have on the production of methylated mercury (the form that accumulates in fish) is unclear. The GEOTRACES program and other work currently underway, is shedding important additional light on the amount and distribution of pollution mercury in the ocean, as well as the factors affecting methylation of mercury. This information, when combined with modelling and anthropogenic emissions estimates will refine our predictions for mercury conditions in the ocean and suggests the best ways to avoid a future of inedible fish. 
Dr Carl Lamborg, University of California, Santa Cruz, USA

Dr Carl Lamborg, University of California, Santa Cruz, USACarl Lamborg gained his BA in Chemistry from Oberlin College, a MS in Environmental Chemistry from the University of Michigan School of Public Health, and his PhD in Oceanography from University of Connecticut. Lamborg worked as Research Assistant at the Harvard School of Public Health and at the University of Connecticut. He was a Postdoctoral Scholar at Woods Hole Oceanographic Institution (WHOI), later becoming Assistant and Associate Scientist at WHOI as well. |
|
| 15:20 - 15:50 |
Anthropogenic Pb isotopes as pollutants and tracers of ocean processes
Anthropogenic emissions of Pb into the atmosphere (dominantly from leaded gasoline consumption, but also and increasingly due to coal combustion, smelting and high temperature industrial processes) have increased the flux of lead into the ocean by about an order of magnitude. Hence most of the Pb in the ocean is anthropogenic. Pb enters the ocean at the surface and then is dispersed by ocean currents and ‘scavenging’ onto (and perhaps release from) sinking particles within the water column. GEOTRACES now has Pb data for at least five sections with at least one from each ocean basin. I will use data on dissolved and particulate Pb concentrations, Pb isotope ratios, and Pb-210 to show how this data – and earlier data showing the temporal evolution of Pb in the ocean – illuminate transport of Pb from different sources through the ocean by currents and sinking particles. In particular, I will demonstrate that most of the variability of particulate Pb in the mid-latitude Atlantic Ocean can be modelled by two-phase partition coefficient reversible exchange, and estimate the extent to which this process alters the distribution of lead from that imposed by the physical circulation of the ocean. 
Professor Ed Boyle, Massachusetts Institute of Technology, USA

Professor Ed Boyle, Massachusetts Institute of Technology, USAProfessor Boyle (PhD, '76, MIT/Woods Hole Oceanographic Institution; BA, '71, University of California, San Diego) is a marine geochemist involved in the study of the natural and anthropogenic metals in the ocean and ice age paleoclimate His research includes studies of past ocean circulation patterns based on the chemistry of microfossils in oceanic sediments, control of late Pleistocene carbon dioxide, and historical trace element variability derived from archives such as corals, sediments and ice cores. He also investigates the trace element chemistry of rivers and estuaries. In particular, he studies the variability of oceanic iron and lead derived from atmospheric transport of anthropogenic emissions and mineral dust, and the evolving fate of atmospherically transported pollutant lead in the ocean. Professor Boyle is a member of the U.S. National Academy of Sciences (2008). |
|
| 16:00 - 16:30 |
The GEOTRACES contribution to the ocean iron fertilisation geoengineering debate
There is increasing focus, by national academies and the IPCC, on evaluating whether geoengineering (also termed climate intervention) can mitigate warming and/or rising atmospheric CO2 concentrations. Of the wide range of Solar Radiation Management and Carbon Dioxide Removal approaches discussed so far, ocean iron fertilisation has been the most thoroughly appraised. This assessment was made possible by the availability of datasets from 12 mesoscale (~1000 km2) ocean iron-enrichment studies, funded to investigate the role of changes in iron supply in altering Earths’ climate in the geological past. The unprecedented scale of these manipulations and their multidisciplinary scope offer penetrating insights into the challenges, benefits, costs and side-effects of geoengineering. Since 2009 GEOTRACES has provided an in-depth study of ocean iron biogeochemistry that has shed light on several unknowns – highlighted in Boyd et al. (2007) Science – that have hindered the debate into iron fertilisation as a geoengineering approach. The GEOTRACES global survey lines have resulted in an improved understanding of iron biogeochemistry in the modern ocean. For example, the discovery of new sources of iron, how they are dispersed, and hence their geographical sphere of influence. GEOTRACES process studies have provided complementary datasets, such as the joint measurement of key properties in the iron and carbon cycles including Fe:C ratios that are valuable to modellers exploring ocean iron fertilisation across a range of scales. In this presentation I will use the findings from the first phase of GEOTRACES to reframe some of the questions central to the ocean geoengineering debate. 
Professor Philip Boyd, Institute for Marine and Antarctic Studies, Australia

Professor Philip Boyd, Institute for Marine and Antarctic Studies, AustraliaPhilip Boyd is a professor of marine biogeochemistry at the Institute for Marine and Antarctic Studies (IMAS) in Hobart, Tasmania. His interests range from the oceans’ iron biogeochemical cycle to the responses of oceanic phytoplankton to climate change. He has served as a member of the GEOTRACES and SOLAS scientific steering committees, and is currently active with the BioGEOTRACES project within GEOTRACES. Additional interests include assessment of the feasibility, side-effects and societal implications of purposefully stimulating marine primary production using iron-enrichment to geoengineer the oceans. |



