Ammonia: zero-carbon fertiliser, fuel and energy store
The production of green ammonia could offer options in the transition to net-zero carbon dioxide emissions
This policy briefing considers the opportunities and challenges associated with the manufacture and future use of zero-carbon or green ammonia.
What is green ammonia?
Ammonia is a pungent gas that is widely used to make agricultural fertilisers. Green ammonia production is where the process of making ammonia is 100% renewable and carbon-free.
One way of making green ammonia is by using hydrogen from water electrolysis and nitrogen separated from the air. These are then fed into the Haber process (also known as Haber-Bosch), all powered by sustainable electricity. In the Haber process, hydrogen and nitrogen are reacted together at high temperatures and pressures to produce ammonia, NH3.
However, the process of making ammonia is currently not a “green” process. It is most commonly made from methane, water and air, using steam methane reforming (SMR) (to produce the hydrogen) and the Haber process. Approximately 90% of the carbon dioxide produced is from the SMR process. This process consumes a lot of energy and produces around 1.8% of global carbon dioxide emissions.
Decarbonisation of ammonia production
Reducing the amount of carbon dioxide produced during the ammonia manufacturing process is critical to achieve net-zero targets by 2050. The best way to reduce carbon emissions when making ammonia is to use low-carbon hydrogen.
The most likely short-term options for creating carbon-free hydrogen at scale are blue hydrogen and green hydrogen:
- Blue hydrogen is where carbon emissions from the steam methane reforming (SMR) process are captured and stored (CCS)
- Green hydrogen is produced using water electrolysis to generate hydrogen and oxygen, using sustainable electricity in the process
Read more about how low-carbon hydrogen is produced at scale (PDF).
What’s the future for green ammonia?
The production of green ammonia could offer further options in the transition to net-zero carbon dioxide emissions. These include:
- Energy storage – ammonia is easily stored in bulk as a liquid at modest pressures (10-15 bar) or refrigerated to -33°C. This makes it an ideal chemical store for renewable energy. There is an existing distribution network, in which ammonia is stored in large refrigerated tanks and transported around the world by pipes, road tankers and ships
- Zero-carbon fuel – ammonia can be burnt in an engine or used in a fuel cell to produce electricity. When used, ammonia’s only by-products are water and nitrogen. The maritime industry is likely to be an early adopter, replacing the use of fuel oil in marine engines
- Hydrogen carrier – there are applications where hydrogen gas is used (e.g. in PEM fuel cells), however hydrogen is difficult and expensive to store in bulk (needing cryogenic tanks or high-pressure cylinders). Ammonia is easier and cheaper to store, and transport and it can be readily “cracked” and purified to give hydrogen gas when required
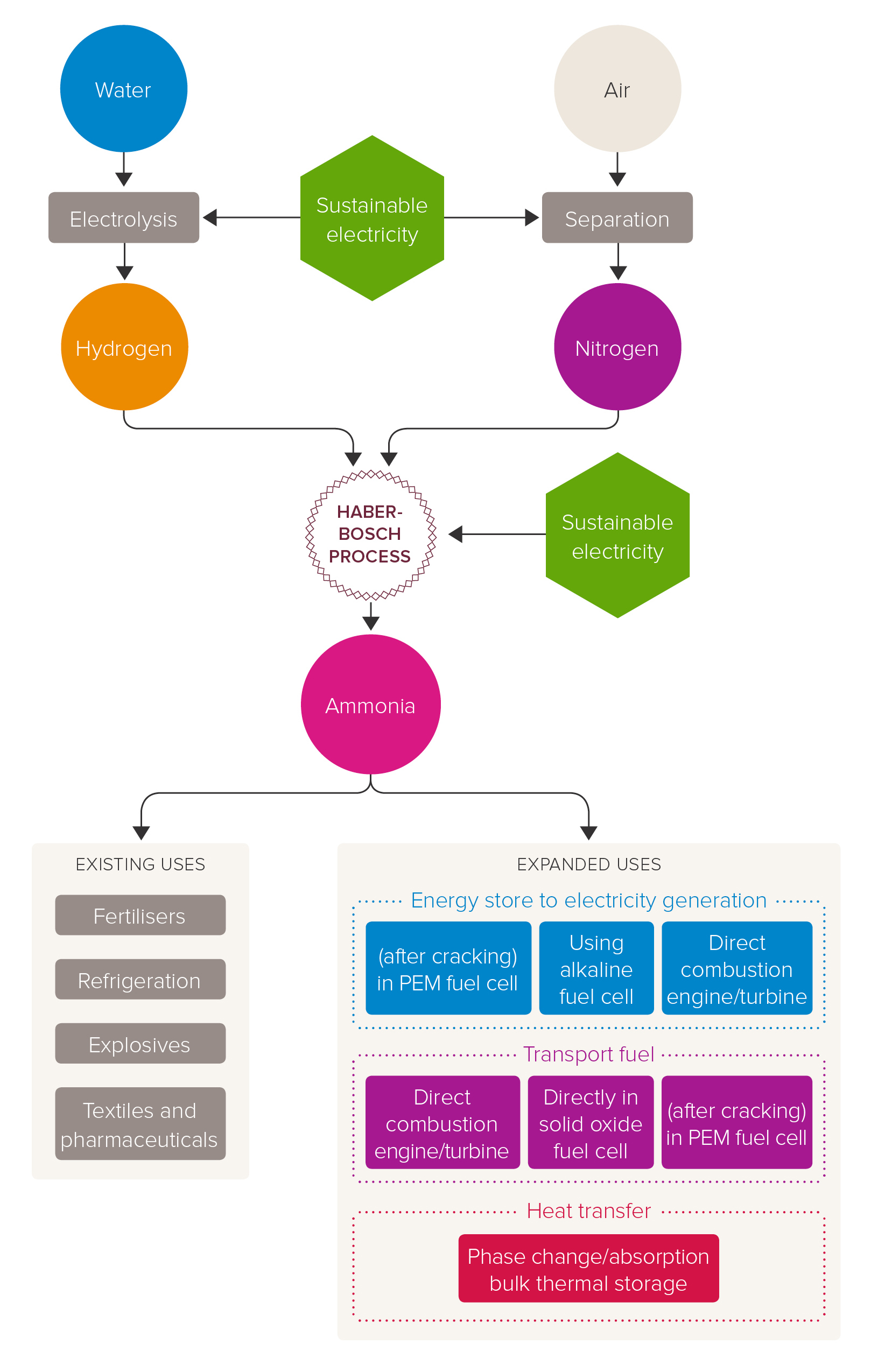
Green ammonia production and use
