A new platform designed by scientists at Gladstone Institutes for studying how the immune system responds to hepatitis C virus could speed the hunt for a vaccine.

Hepatitis C virus (HCV) remains a global public health challenge. An estimated 71 million people are chronically infected, with surges in new cases and no effective preventative. The virus works by primarily targeting the liver. After initial infection, some people recover with few or no symptoms, but others experience lifelong infection that can lead to severe liver disease. Medications can successfully treat HCV but can be difficult to access or afford, and a person who has been treated can later be reinfected.
A vaccine has eluded scientists for more than 30 years, for several reasons. For one, the virus that causes the disease comes in many genetic forms, complicating the creation of a widely effective vaccine. For another, studying hepatitis C has been difficult because options in animals are limited and lab methods using infected cells have not adequately reflected the real-life dynamics of infection.
Now, researchers at Gladstone Institutes have developed a new platform for studying how the human immune system responds to HCV infection. The method, presented in Open Biology, marries microfluidic technology (which allows scientists to precisely manipulate fluid at a microscopic scale) with liver organoids (three-dimensional cell clusters that mimic the biology of real human livers). We asked one of the lead authors and Senior Investigator Melanie Ott (Gladstone Institute of Virology and Immunology) to tell us more about the team’s discovery.
What is your article about?
Our article establishes a novel co-culture system to understand immune responses to Hepatitis C infection using CD8+ T cells and liver organoids grown from HCV+ patients. We find that CD8+ T cells are able to recognise HCV antigen on the surface of the organoids and specifically kill these organoids. This sets up a quantitative platform for further research into immune responses to HCV, such as understanding potential vaccination strategies.
What was most surprising about the method used in the study?
We were concerned that the different media requirements between the T cells and organoids would prevent a coculture system from being effective, and were pleased to see that within the time frame of the study the T cells were able to maintain their viability and function when cultured under in organoid media. 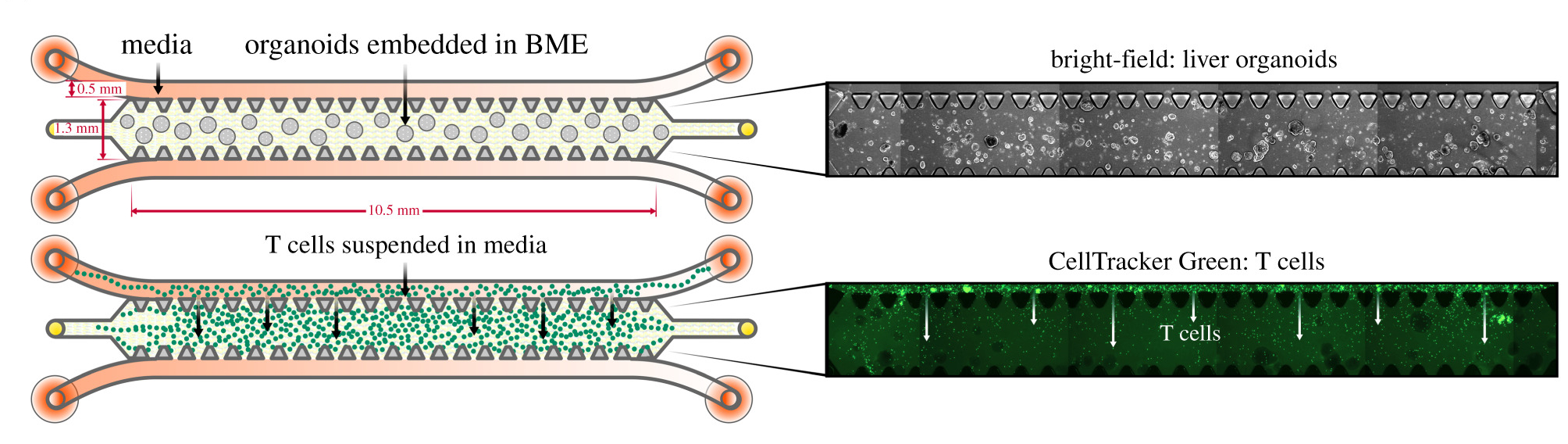
Your manuscript was invited by our Preprint Editorial Team, what was your experience publishing via this route?
We had a great experience publishing via this route. It was simple to transfer our manuscript over from bioRxiv, and we had a speedy review process and found the editors very responsive.
What's next for you and your group’s research?
We have spent the past two years pivoting part of the lab work to SARS-CoV-2 research and are excited to adapt this platform to SARS-CoV-2+ airway organoids and immune cells to understand the cytokine response that leads to severe COVID-19.
.jpg)
Do you have an exciting new discovery that you would like to publish in Open Biology? Find out more about our author benefits and submission process.
Image credits:
Schematic detailing the experimental setup for monoculture of organoids and T cells, individually, in 3D microfluidic chips with corresponding microscopy images. (Figure 3: doi.org/10.1098/rsob.210320)
Group photo: Ott Lab group, Gladstone Institute of Virology and Immunology (Credit: Gladstone Institutes)




