A recent study in Open Biology investigates the distinct roles of glutamate transporters in retinal signal processing and bipolar cell responses in zebrafish.
In the nervous system of vertebrates, glutamate is a key chemical that helps send signals between nerve cells. But in the retina, glutamate acts differently. Instead of exciting the next nerve cells, it actually makes them less active through a process called a ‘glutamate-gated chloride current’. This might seem surprising since glutamate usually stimulates nerve cells. We asked first author Stephan Neuhauss to tell us more about this seemingly paradoxical event and explain why there's still so much to learn about the brain and the retina.
Please could you summarise the main findings of your study
In our study we could demonstrate the involvement of glutamate transporters in direct synaptic transmission between light sensing photoreceptors and downstream bipolar cells of the outer retina. Traditionally glutamate transporters are seen as proteins that remove glutamate, the main excitatory neurotransmitter of the vertebrate nervous system, from the synaptic cleft in order to allow subsequent transmission events. Additionally, excess of glutamate around synapses is linked to neuronal cell damage in a process termed excitotoxicity. Glutamate transporters (EAAT, excitatory amino acid transporters) are very peculiar in that they not only transport extracellular glutamate into cells, but at the same time also allow chloride ions to slip to cross the membrane. Such an inward flux of chloride is typically associated with inhibitory neurotransmission, for instance mediated by ionotropic GABA receptors. It was already well established that glutamate transporters present on presynaptic neurons can provide a negative feedback mechanism that lowers the membrane potential of excited neurons.
From pharmacological experiments there was already some hints that glutamate transporters may be involved in direct synaptic transmission between photoreceptor and ON-bipolar cells. These bipolar cells are special since paradoxically they are inhibited (lower their membrane potential) in response to glutamate binding. We therefore characterized all eaat genes in the zebrafish that code for glutamate transporters. Two of the many (11 in total) are located postsynaptically on ON-bipolar cells, hence in the right spot to mediate synaptic transmission.

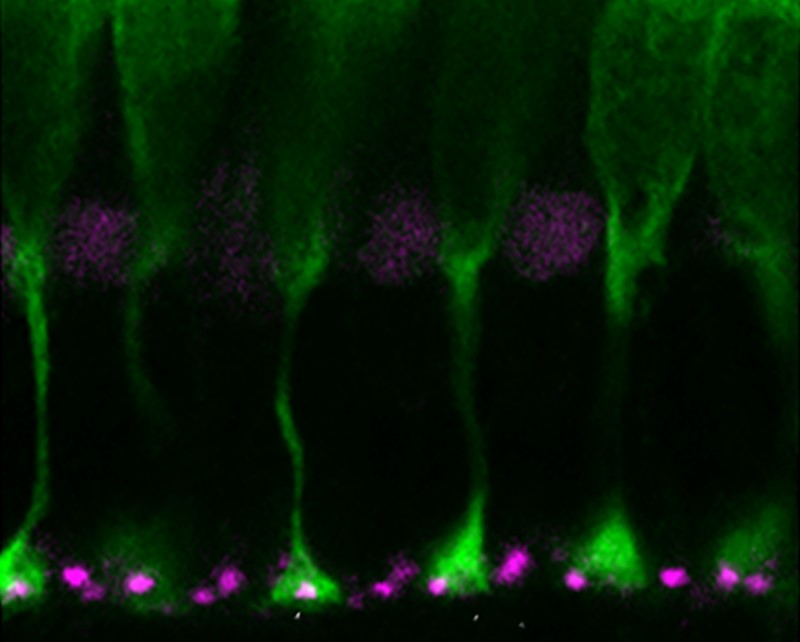
Glutamate transporters eaat5b and eaat7 mRNA is detected in the INL of the retina: doi.org/10.1098/rsob.240140
After verifying in biophysical experiment that they indeed induce a chloride current after glutamate binding, we generated mutants in these two genes and tested their influence on vision. We did this by recording light induced neuronal responses in the outer retina using electroretingraphy (ERG). Indeed, we found alterations in the response, both in strength and kinetics, indicating that our hypothesis is indeed true. Nevertheless, the eyes could still respond to light, indicating that another well-established mechanism using a metabotropic glutamate receptor is a major component of the light response. Therefore, this receptor (mGluR6) works in collaboration with the glutamate transporters.
What challenges did you encounter in generating and analysing the zebrafish models? Did any other challenges arise?
When we started this project, we were surprised how many eaat genes we found in the fish genome in contrast to mammalian systems (11 in fish, 5 in mammals). This led to another interesting evolutionary study documenting clade specific gene loss. The advent of genome editing made the generation of the mutants quite efficient, but to proof protein localization to the postsynaptic membrane was a major challenge. We had to develop our own antibodies that would also need to be specific between the closely related isoforms of this gene family.
What are the potential implications of your findings for understanding visual processing in other vertebrates, including humans?
For evolutionary reasons that may be related to the “reinvention” of colour vision in some mammals, the elegant glutamate transporter mechanism probably plays a more prominent role in non-mammalian vertebrates. Since the main mediator in the ON-pathway is likely the metabotropic glutamate receptors in mammals, supported by a number of genetic diseases linked leading to congenital stationary blindness, the role of glutamate receptors are likely modulatory.
What are the next steps in your research?
In future experiments, we would like to uncover their specific role. For one member we have preliminary evidence for a role in stabilising the resting potential of photoreceptors. The absence of this transport leads to a dramatic reduction of temporal resolution of vision.
---------------------------------------------------------------------------------------------------Do you have an exciting new discovery that you would like to publish in Open Biology? Find out more about our author benefits and submission process.
Thumbnail image: Blue cones and EAAT. Credit – Stephan Neuhauss




