A new Royal Society Open Science study investigates how the Photorhabdus virulence cassette (PVC), a naturally occurring protein delivery system, selects and loads its payload proteins for injection into eukaryotic cells. This discovery opens exciting possibilities for novel drug delivery mechanisms. We spoke to Dr Rhys Evans at the University of Warwick to find about more about the work.
Many protein-based drugs target cell surface receptors to alter cell behaviour. For cancers, for example, treatments might focus on reprogramming diseased cells to make them more vulnerable to the immune system. The PVC offers an alternative solution by delivering therapeutic proteins directly into the cell’s interior – a game changer in the field of intracellular medicine.
We studied the PVC: a naturally occurring bacterial protein delivery device which targets eukaryotic cells by injecting its payload into the cytosol. Ordinarily, PVCs and their naturally occurring payloads are used to enhance infection, but with some bioengineering and the ability to target the cell interior, the PVC could revolutionize the delivery of protein-based drugs by influencing cellular processes from within.
Summary of the study
Using E. coli, we were able to express and purify PVCs to see how they can select and load their payloads into the PVC’s hollow tube lumen. Previous studies had characterised signal peptides (SPs) found at the N-terminus of some putative PVC payload proteins which were shown to be important for PVC loading. These SPs though hard to characterize due to low sequence homology, are typically predicted as disordered regions in protein structure models.
However, we identified that the PVC isn’t the only one of its kind to employ SPs for loading. The PVC is one type of extracellular contractile injection system (eCIS); we found that many of its eCIS homologues have putative payloads which also possess SPs. This finding suggests that SPs are a common mechanism for loading payload proteins across eCIS, but also points to potential SP-independent loading mechanisms in other species – an area ripe for further exploration.
A key focus of the study was on a non-structural component of the PVC, Pvc15, which we suspected might be responsible for the ATP-driven loading of SP-tagged proteins into the PVC. Using bioinformatics, we identified Pvc15 as an ‘ATPase associated with diverse cellular activities’ (AAA), possessing two ATPase domains. We set out to determine if both domains are functional ATPases, and whether ATPase activity was required for loading SP-tagged payloads into the PVC.
Through targeted gene knockouts of Pvc15, we generated complete PVCs but which, unlike the wild-type, lacked SP-tagged payloads. Additionally, we found that shortening the SP – despite stabilizing the payloads – prevented loading, confirming the crucial role of the SP in the process.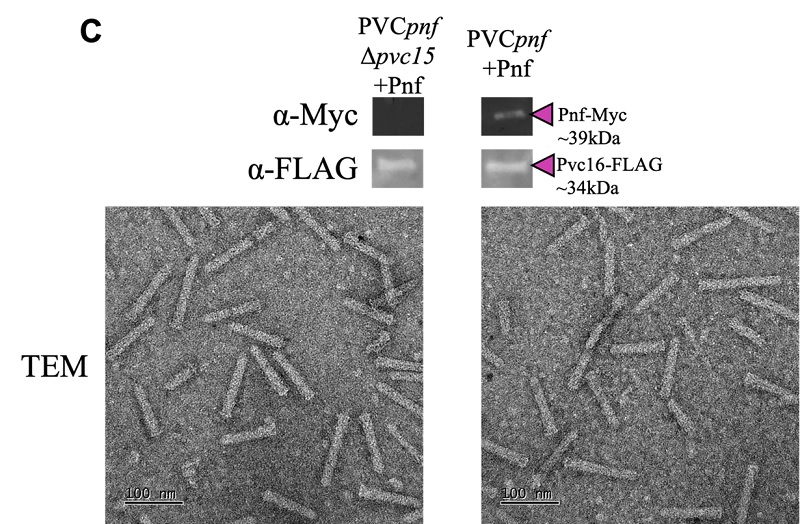
Transmission electron microscope (TEM) images show intact PVCs. A gene knockout of pvc15 results in hollow PVCs with no associated payload (Pnf).
Interestingly, empty PVCs did not affect the respiration of human immune cell cultures in vitro, suggesting that the injection system alone is non-toxic to human cells. This indicates that toxicity requires both the PVC and its associated payload, further emphasizing the importance of successful payload loading.
To explore how Pvc15 facilitates payload loading, we created custom plasmids encoding SP-tagged payloads alongside mutant strains of Pvc15. By purifying these PVCs, we investigated the role of Pvc15's ATPase activity in the loading process. Remarkably, disrupting ATPase activity in just one of the two ATPase domains abolished payload loading, despite increased stability of the SP-tagged proteins in the bacteria.
This crucial finding highlights that ATPase activity in Pvc15 is essential for loading the payload into the PVC, potentially by "pumping" the payload into the PVC tube or associating it with structural proteins during assembly.
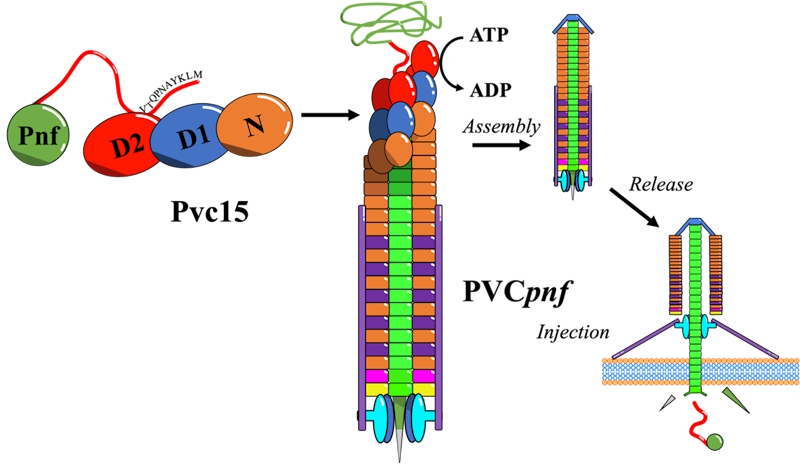
Pnf payload into the PVC nanostructure during assembly. After being released from the bacterium, the PVC can bind and inject this payload into a target cell.
What are the implications of your findings?
Our research offers a major step forward in understanding how PVCs can be engineered as synthetic protein delivery devices. By identifying the critical role of Pvc15 and SPs in payload loading, we’re one step closer to optimizing this naturally occurring system for therapeutic applications.
This study, part of my PhD work, was conducted in collaboration with Nanosyrinx Ltd: a university spin-out company working to harness PVCs for intracellular drug delivery. Their recent funding and expansion have accelerated efforts to test these devices in vivo, bringing us closer to a new era of intracellular medicine that could "hack" human cells from within.
What are the next steps?
Looking ahead, there are several promising avenues for further research. One exciting direction is exploring the specificity between Pvc15 and SPs. Do different Pvc15 homologues selectively interact with specific SP sequences? Investigating the potential co-evolution of Pvc15 and SPs could yield fascinating insights into this protein delivery mechanism.
Another key area is understanding how payload proteins refold after being injected into the target cell, as we believe they are unfolded during the loading process. This aspect remains one of the most elusive mysteries in the field of eCIS research and could hold the key to fully unlocking the therapeutic potential of PVCs.
Finally, retargeting the PVCs to inject payloads into specific cell types is another critical frontier. Some research teams have already made progress in this area, and building on these successes is crucial to ensure that therapeutic payloads are delivered precisely to the cells where they are most needed. Achieving targeted delivery would significantly enhance the efficacy and safety of PVC-based therapies, bringing us closer to clinical applications.
By continuing to investigate these questions, we aim to refine the PVC system for use in human medicine and open new possibilities for targeted intracellular therapies.
Royal Society Open Science is an open access journal that welcomes the submission of all high-quality science. More information about the journal and the submission process can be found on our webpage.
Image 1. Scientific image of bacteria Bacteroides fragilis and other Bacteroides. Dr_Microbe.
Image 2. Transmission electron microscope (TEM) images show intact PVCs. A gene knockout of pvc15 results in hollow PVCs with no associated payload (Pnf).
Image 3: Pvc15 may translocate the SP-tagged Pnf payload into the PVC nanostructure during assembly. After being released from the bacterium, the PVC can bind and inject this payload into a target cell.





